Page 50 - IJB-8-1
P. 50
Controlling Droplet Impact Velocity and Droplet Volume Improves Cell Viability in Droplet-Based Bioprinting
A
B
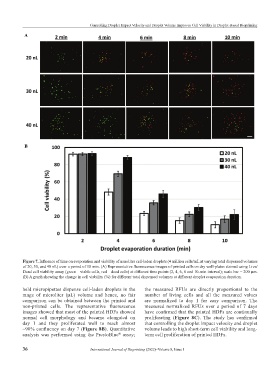
Figure 7. Influence of time on evaporation and viability of nanoliter cell-laden droplets (4 million cells/mL at varying total dispensed volumes
of 20, 30, and 40 nL) over a period of 10 min. (A) Representative fluorescence images of printed cells on dry well-plates stained using Live/
Dead cell viability assay (green – viable cells, red – dead cells) at different time points (2, 4, 6, 8 and 10-min interval); scale bar = 200 µm.
(B) A graph showing the change in cell viability (%) for different total dispensed volumes at different droplet evaporation duration.
held micropipettes dispense cell-laden droplets in the the measured RFUs are directly proportional to the
range of microliter (µL) volume and hence, no fair number of living cells and all the measured values
comparison can be obtained between the printed and are normalized to day 1 for easy comparison. The
non-printed cells. The representative fluorescence measured normalized RFUs over a period of 7 days
images showed that most of the printed HDFs showed have confirmed that the printed HDFs are continually
normal cell morphology and became elongated on proliferating (Figure 8C). The study has confirmed
day 1 and they proliferated well to reach almost that controlling the droplet impact velocity and droplet
~90% confluency on day 7 (Figure 8B). Quantitative volume leads to high short-term cell viability and long-
analysis was performed using the PrestoBlue assay; term cell proliferation of printed HDFs.
®
36 International Journal of Bioprinting (2022)–Volume 8, Issue 1