Page 139 - IJB-8-2
P. 139
Li, et al.
A B
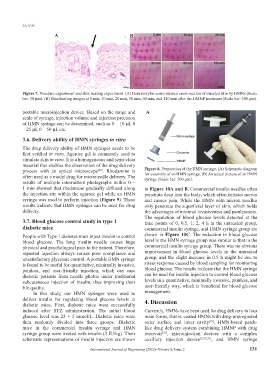
Figure 7. Puncture experiment and skin healing experiment. (A) Hematoxylin-eosin-stained cross-section of inserted skin by HMNs (Scale
bar: 50 μm). (B) Skin healing images at 0 min, 10 min, 20 min, 30 min, 60 min, and 120 min after the HMNP treatment (Scale bar: 500 μm).
portable microinjection device. Based on the range and A B
scale of syringe, injection volume and injection precision
of HMN syringe can be determined, such as 0 – 10 μl, 0
– 25 μl, 0 – 50 μl, etc.
3.6. Delivery ability of HMN syringes in vitro
The drug delivery ability of HMN syringes needs to be
first verified in vitro. Agarose gel is commonly used to
simulate skin in vitro. It is a homogeneous and semi-clear
material that enables the observation of the drug delivery
process with an optical microscope . Rhodamine is Figure 8. Preparation of the HMN syringe. (A) Schematic diagram
[27]
for assembly of an HMN syringe. (B) An actual picture of an HMN
often used as a model drug for microneedle delivery. The syringe (Scale bar: 500 μm).
results of multiple intermittent photographs within 0 –
1 min showed that rhodamine gradually diffused along in Figure 10A and B. Commercial insulin needles often
the injection site within the agarose gel while an HMN penetrate deep into the body, which often irritates nerves
syringe was used to perform injection (Figure 9). These and causes pain. While the HMN with micron needles
results indicate that HMN syringes can be used for drug only penetrate the superficial layer of skin, which holds
delivery. the advantages of minimal invasiveness and painlessness.
The regulation of blood glucose levels detected at the
3.7. Blood glucose control study in type 1 time points of 0, 0.5, 1, 2, 4 h in the untreated group,
diabetic mice commercial insulin syringe, and HMN syringe group are
People with Type 1 diabetes must inject insulin to control shown in Figure 10C. The reduction in blood glucose
blood glucose. The long insulin needle causes huge level in the HMN syringe group was similar to that in the
physical and psychological pain to the patient. Therefore, commercial insulin syringe group. There was no obvious
repeated injection always causes poor compliance and improvement in blood glucose levels in the untreated
unsatisfactory glycemic control. A portable HMN syringe group, and the slight increase in 0.5 h might be due to
is found to be useful for quantitative, minimally invasive, stress response caused by blood sampling for monitoring
painless, and user-friendly injection, which can ease blood glucose. The results indicate that the HMN syringe
diabetic patients from needle phobia under traditional can be used for insulin injection to control blood glucose
subcutaneous injection of insulin, thus improving their levels in a quantitative, minimally invasive, painless, and
life quality. user-friendly way, which is beneficial for blood glucose
In this study, our HMN syringes were used to management.
deliver insulin for regulating blood glucose levels in 4. Discussion
diabetic mice. First, diabetic mice were successfully
induced after STZ administration. The initial blood Currently, HMNs have been used for drug delivery in four
glucose level was 23 ± 5 mmol/L. Diabetic mice were main forms, that is, coated HMNs with drug-impregnated
then randomly divided into three groups. Diabetic outer surface and inner cavity , HMN-based patch-
[29]
mice in the commercial insulin syringe and HMN like drug delivery system combining HMNP with drug
syringe group were treated with insulin (2 IU/kg). Their reservoir , microinjection devices with a complex
[32]
schematic representations of insulin injection are shown auxiliary injection device [5,7,8,33] , and HMN syringe
International Journal of Bioprinting (2022)–Volume 8, Issue 2 131