Page 138 - IJB-8-2
P. 138
3D Printing of Hollow Microneedle Patches
without layer-by-layer structure. As a result, SOPL distance of about 250 μm and compression strength of
technology enables rapid customization of high-quality about 4 N. It was reported that insertion forces of 0.1 –
HMNPs. 3 N were sufficient to permit insertion by hand . This
[31]
reveals that the mechanical strength of HMNs is sufficient
3.2. In vitro biocompatibility of HMNPs for skin puncture.
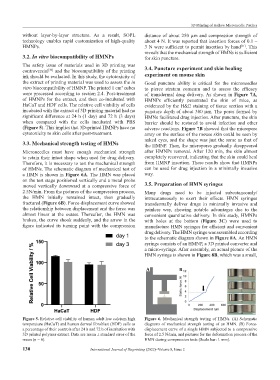
The safety issue of materials used in 3D printing was 3.4. Puncture experiment and skin healing
controversial and the biocompatibility of the printing
[30]
ink should be evaluated. In this study, the cytotoxicity of experiment on mouse skin
the extract of printing material was used to assess the in Good puncture ability is critical for the microneedles
vitro biocompatibility of HMNP. The printed 1 cm cubes to pierce stratum corneum and to assess the efficacy
3
were processed according to section 2.4. Post-treatment of transdermal drug delivery. As shown in Figure 7A,
of HMNPs for the extract, and then co-incubated with HMNPs efficiently penetrated the skin of mice, as
HaCaT and HDF cells. The relative cell viability of cells evidenced by the H&E staining of tissue section with a
incubated with the extract of 3D printing material had no puncture depth of about 300 μm. The pores formed by
significant difference at 24 h (1 day) and 72 h (3 days) HMNs facilitated drug injection. After puncture, the skin
when compared with the cells incubated with PBS barrier should be restored to avoid infection and other
(Figure 5). This implies that 3D-printed HMNPs have no adverse reactions. Figure 7B showed that the micropore
cytotoxicity to skin cells after post-treatment. array on the surface of the mouse skin could be seen by
naked eyes, and the shape was just the same as that of
3.3. Mechanical strength testing of HMNs the HMNP. Then, the micropores gradually disappeared
Microneedles must have enough mechanical strength after HMNPs removal. After 120 min, the skin almost
to retain their intact shape when used for drug delivery. completely recovered, indicating that the skin could heal
Therefore, it is necessary to test the mechanical strength from HMNP insertion. These results show that HMNPs
of HMNs. The schematic diagram of mechanical test of can be used for drug injection in a minimally invasive
a HMN is shown in Figure 6A. The HMN was placed way.
on the test stage positioned vertically and a metal probe
moved vertically downward at a compressive force of 3.5. Preparation of HMN syringes
2.5N/min. From the pictures of the compression process, Many drugs need to be injected subcutaneously/
the HMN initially remained intact, then gradually intracutaneously to exert their effects. HMN syringes
fractured (Figure 6B). Force-displacement curve showed transdermally deliver drugs in minimally invasive and
the relationship between displacement and the force was painless way, showing notable advantages due to the
almost linear at the outset. Thereafter, the HMN was convenient quantitative delivery. In this study, HMNPs
broken, the curve shook suddenly, and the arrow in the with holes at the bottom (Figure 3C) were used to
figure indicated its turning point with the compression manufacture HMN syringes for efficient and convenient
drug delivery. The HMN syringe was assembled according
to the schematic diagram shown in Figure 8A. An HMN
syringe consists of an HMNP, a 3D printed converter and
a micro-syringe. After assembly, an actual picture of the
HMN syringe is shown in Figure 8B, which was a small,
A B
Figure 5. Relative cell viability of human adult low calcium high Figure 6. Mechanical strength testing of HMNs. (A) Schematic
temperature (HaCaT) and human dermal fibroblast (HDF) cells as diagrams of mechanical strength testing of an HMN. (B) Force-
a percentage of their controls after 24 h and 72 h of incubation with displacement curve of a single HMN subjected to a compressive
3D printed polymer extract. Data are mean ± standard error of the force of 2.5 N/min, and pictures for the deformation process of the
mean (n = 6). HMN during compression tests (Scale bar: 1 mm).
130 International Journal of Bioprinting (2022)–Volume 8, Issue 2