Page 151 - IJB-8-2
P. 151
Kanaki, et al.
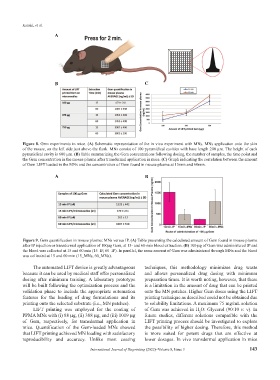
A
B C
Figure 8. Gem experiments in mice. (A) Schematic representation of the in vivo experiment with MNs. MNs application onto the skin
of the mouse, on the left side just above the flank. MNs consist of 100 pyramidical cavities with base length 200 μm. The height of each
pyramidical cavity is 600 μm. (B) Table summarizing the Gem concentrations following dosing, the number of samples, the time point and
the Gem concentration in the mouse plasma after transdermal application in mice. (C) Graph indicating the correlation between the amount
of Gem LIFT loaded in the MNs and the concentration of Gem found in mouse plasma at 15min and 60min.
A B
Figure 9. Gem quantification in mouse plasma: MNs versus IP. (A) Table presenting the calculated amount of Gem found in mouse plasma
after IP injection or transdermal application of 100μg Gem, at 15- and 60-min blood extraction. (B) 100 μg of Gem was administered IP and
the blood was collected at 15 and 60 min (15_IP, 60_IP). In parallel, the same amount of Gem was administered through MNs and the blood
was collected at 15 and 60 min (15_MNs, 60_MNs).
The automated LIFT device is greatly advantageous techniques, this methodology minimizes drug waste
because it can be used by medical staff offer personalized and allows personalized drug dosing with minimum
dosing after minimum training. A laboratory prototype preparation times. It is worth noting, however, that there
will be built following the optimization process and the is a limitation in the amount of drug that can be printed
validation phase to include the appropriate automation onto the MN patches. Higher Gem doses using the LIFT
features for the loading of drug formulations and its printing technique as described could not be obtained due
printing onto the selected substrate (i.e., MN patches). to solubility limitations. A maximum 75 mg/mL solution
LIFT printing was employed for the coating of of Gem was achieved in H O: Glycerol (90:10 v: v). In
2
PPMA MNs with (i) 88 μg, (ii) 388 μg, and (iii) 1019 μg future studies, different solutions compatible with the
of Gem, respectively, for transdermal application in LIFT printing process should be investigated to explore
mice. Quantification of the Gem-loaded MNs showed the possibility of higher dosing. Therefore, this method
that LIFT printing achieved MN loading with satisfactory is more suited for potent drugs that are effective at
reproducibility and accuracy. Unlike most coating lower dosages. In vivo transdermal application in mice
International Journal of Bioprinting (2022)–Volume 8, Issue 3 143