Page 16 - IJB-8-3
P. 16
Ultrathin Scaffolds for Monolayer RPE Cell Culture
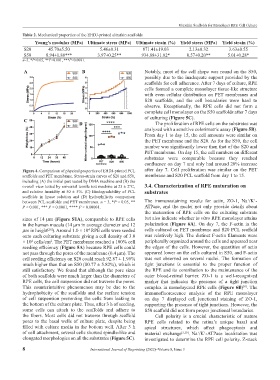
Table 2. Mechanical properties of the EHDJ-printed ultrathin scaffolds
Young’s modulus (MPa) Ultimate stress (MPa) Ultimate strain (%) Yield stress (MPa) Yield strain (%)
S20 45.70±5.50 5.46±0.31 871.41±19.03 2.13±0.32 3.63±0.55
S50 8.94±1.88*** 3.97±0.25** 934.88±31.82* 0.57±0.20** 5.01±0.28*
n=3, *P<0.05, **P<0.001, ***P<0.0001.
A B Notably, most of the cell shape was round on the S50,
possibly due to the inadequate support provided by the
scaffolds for cell adherence. After 7 days of culture, RPE
cells formed a complete monolayer tissue-like structure
with even cellular distribution on PET membranes and
S20 scaffolds, and the cell boundaries were hard to
observe. Exceptionally, the RPE cells did not form a
complete cell monolayer on the S50 scaffolds after 7 days
of culturing (Figure 5C).
C
D The proliferation of RPE cells on the substrates was
analyzed with a sensitive colorimetric assay (Figure 5B).
From day 1 to day 15, the cell amounts were similar on
the PET membrane and the S20. As for the S50, the cell
number was significantly lower than that of the S20 and
PET membrane. On day 15, the cell numbers on different
substrates were comparable because they reached
confluence on day 7 and only had around 20% increase
Figure 4. Comparison of physical properties of EHDJ-printed PCL after day 7. Cell proliferation was similar on the PET
scaffolds and PET membrane. Stress-strain curves of S20 and S50, membrane and S20 PCL scaffold from day 1 to 15.
including (A) the initial part tested by DMA machine and (B) the
overall view tested by universal tensile test machine at 25 ± 2°C, 3.4. Characterization of RPE maturation on
and relative humidity at 50 ± 5%. (C) Biodegradability of PCL substrates
scaffolds in lipase solution and (D) hydrophilicity comparison
+
+
between PCL scaffolds and PET membranes. n = 3, *P < 0.05, ** The immunostaining results for actin, ZO-1, Na /K -
P < 0.001, *** P < 0.0001, **** P < 0.00001. ATPase, and the nuclei not only provide details about
the maturation of RPE cells on the culturing substrate
sizes of 14 μm (Figure S1A), comparable to RPE cells but also indicate whether in vitro RPE monolayer attains
in the human macula (14 μm in average diameter and 12 polarization (Figure 6A). On day 7, the F-actin in the
μm in height ). Around 1.0 × 10 RPE cells were seeded cells cultured on PET membrane and S20 PCL scaffold
[28]
4
onto each culturing substrate giving a cell density of 3.0 was relatively high. The distinct F-actin filaments were
× 10 cells/cm . The PET membrane reached a 100% cell peripherally organized around the cells and appeared near
4
2
seeding efficiency (Figure 5A) because RPE cells could the edges of the cells. However, the quantities of actin
not pass through the pores of the membrane (0.4 μm). The appeared lower on the cells cultured in S50, and F-actin
cell seeding efficiency on S20 could reach 92.87 ± 1.96% was not observed on several nuclei. The formation of
much higher than that on S50 (80.77 ± 5.82%), which is tight junctions is essential to the proper function of
still satisfactory. We found that although the pore sizes the RPE and its contribution to the maintenance of the
of both scaffolds were much larger than the diameters of outer blood-retinal barrier. ZO-1 is a well-recognized
RPE cells, the cell suspension did not traverse the pores. marker that indicates the presence of a tight junction
This counterintuitive phenomenon may be due to the complex in monolayered RPE cells (Figure 6B) . The
[6]
hydrophobicity of the scaffolds and the surface tension immunofluorescence analysis of the RPE monolayer
of cell suspension preventing the cells from leaking to on day 7 displayed cell junctional staining of ZO-1,
the bottom of the culture plate. Thus, after 3 h of seeding, supporting the presence of tight junctions. However, the
some cells can attach to the scaffolds and adhere to S50 scaffold did not form proper junctional boundaries.
the fibers. Most cells did not traverse through scaffold Cell polarity is a crucial characteristic of mature
pores to the basal wells of culture plate, despite being RPE cells related to the retina’s unique basal and
filled with culture media in the bottom well. After 3 h apical structures, which affect phagocytosis and
of cell attachment, several cells showed spindle-like and material exchange [5,10] . Na /K -ATPase localization was
+
+
elongated morphologies on all the substrates (Figure 5C). investigated to determine the RPE cell polarity. Z-stack
8 International Journal of Bioprinting (2022)–Volume 8, Issue 3