Page 72 - IJB-8-3
P. 72
Extrusion of two-vasculature scaffold for angiogenesis
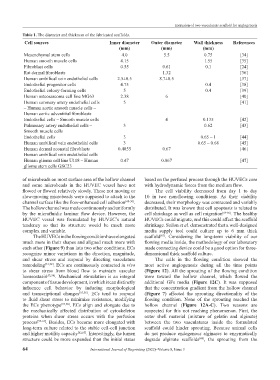
Table 1. The diameter and thickness of the fabricated scaffolds.
Cell sources Inner diameter Outer diameter Wall thickness References
(mm) (mm) (mm)
Mesenchymal stem cells 4.0 5.5 0.75 [34]
Human smooth muscle cells 4.15 1.55 [35]
Fibroblast cells 0.55 0.61 0.1 [24]
Rat dermal fibroblasts 1.32 [36]
Human umbilical vein endothelial cells 2.5±0.5 8.7±0.5 [37]
Endothelial progenitor cells 4.75 0.4 [38]
Endothelial colony-forming cells 5 0.4 [39]
Human osteosarcoma cell line MG63 2.38 6 [40]
Human coronary artery endothelial cells 5 [41]
– Human aortic smooth muscle cells –
Human aortic adventitial fibroblasts
Endothelial cells – Smooth muscle cells 4 0.135 [42]
Pulmonary artery endothelial cells – 3 0.62 [43]
Smooth muscle cells
Endothelial cells 3 0.65 – 1 [44]
Human umbilical vein endothelial cells 3 0.65 – 0.68 [45]
Human dermal neonatal fibroblasts – 0.4855 0.67 [46]
Human umbilical vein endothelial cells
Human glioma cell line U118 – Human 0.47 0.867 [47]
glioma stem cells GSC23
of microbeads on most surface area of the hollow channel based on the perfused process through the HUVECs core
and some microbeads in the HUVEC vessel have not with hydrodynamic forces from the medium flow.
flowed or flowed relatively slowly. These not moving or The cell viability decreased from day 1 to day
slow-moving microbeads were supposed to attach to the 10 in two non-flowing conditions. As their viability
channel surface like the flow-enhanced cell adhesion [48-50] . decreased, their morphology was contracted and variably
The hollow channel was made continuously and uniformly distributed. It was known that cell apoptosis is related to
by the microfluidic laminar flow device. However, the cell shrinkage as well as cell migration [64-66] . The healthy
HUVEC vessel was formulated by HUVEC’s natural HUVECs could migrate, and this could affect the scaffold
tendency so that its structure would be much more shrinkage. Sailon et al. demonstrated that a well-designed
complex and variable. media supply tool could culture up to 6 mm thick
The HUVECs in the flowing condition have elongated scaffold . Considering the long-term viability of our
[67]
much more in their shapes and aligned much more with flowing media inside, the methodology of our laboratory
each other (Figure 9) than into two other conditions. ECs made connecting device could be a good option for three-
recognize minor variations in the direction, magnitude, dimensional thick scaffold culture.
and shear stress and respond by directing vasculature The cells in the flowing condition showed the
remodeling [51,52] . ECs are continuously contacted in vivo most active angiogenesis during all the time points
to shear stress from blood flow to maintain vascular (Figure 12). All the sprouting of the flowing condition
homeostasis [53,54] . Mechanical stimulation is an integral were toward the hollow channel, which flowed the
component of tissue development, in which it can distinctly additional GFs media (Figure 12C). It was supposed
influence cell behavior by inducing morphological that the concentration gradient from the hollow channel
and transcriptional changes [55,56] . ECs tend to respond (Figure 7) affected the sprouting directionality of the
to fluid shear stress to minimize resistance, modifying flowing condition. None of the sprouting reached the
the ECs phenotype [57,58] . ECs align and elongate due to hollow channel (Figure 12A-C). Two reasons are
the mechanically affected distribution of cytoskeleton suspected for this not reaching phenomenon. First, the
proteins when shear stress occurs with the perfusion outer shell material (mixture of gelatin and alginate)
process [59-61] . Besides, ECs become more elongated with between the two vasculatures inside the formulated
long-term culture related to the stable cell-cell junction scaffold could hinder sprouting. Because animal cells
and higher motility capacity [62,63] . Interestingly, the lumen do not produce endogenous alginases to enzymatically
structure could be more expanded than the initial status degrade alginate scaffolds , the sprouting from the
[68]
64 International Journal of Bioprinting (2022)–Volume 8, Issue 3