Page 99 - IJB-8-4
P. 99
Soman, et al.
A B C J
D E F
K
G H I
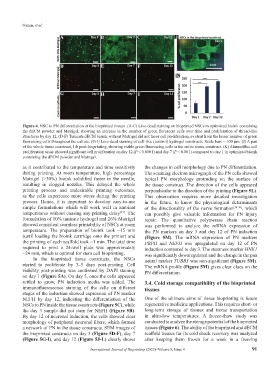
Figure 4. NSC to PN differentiation of the bioprinted tissues. (A-C) Live-dead staining on bioprinted NSCs in optimized bioink containing
the dECM powder and Matrigel, showing an increase in the number of green florescent cells over time and proliferation of thread-like
structures by day 12. (D-F) Tunicate dECM bioink without Matrigel did not favor cell proliferation, evident from the lesser number of green
fluorescing cells throughout the culture. (G-I) Live-dead staining of cell-free (control) hydrogel constructs. Scale bars = 100 µm. (J) A part
of the whole tissue construct, 1-h post-bioprinting, showing viable green fluorescing cells in the entire tissue construct. (K) AlamarBlue cell
proliferation assay showed significant cell proliferation on day 12 (P < 0.0001) and day 7 (P < 0.001) compared to day 1 in optimized bioink
containing the dECM powder and Matrigel.
as it contributed to the temperature and time sensitivity the changes in cell morphology due to PN differentiation.
during printing. At room temperature, high percentage The scanning electron micrograph of the PN cells showed
Matrigel (>30%) bioink solidified faster in the needle, typical PN morphology protruding on the surface of
resulting in clogged nozzles. This delayed the whole the tissue construct. The direction of the cells appeared
printing process and undesirable printing outcomes, perpendicular to the direction of the printing (Figure 5L).
as the cells experience more stress during the printing This observation requires more detailed investigation
process. Hence, it is important to develop easy-to-use in the future, to know the physiological determinants
simple formulations which will work well in ambient of the directionality of the nerve formation [34-36] , which
temperatures without causing any printing delay . The can possibly give valuable information for PN injury
[33]
formulation of 10% tunicate hydrogel and 26% Matrigel repair. The quantitative polymerase chain reaction
showed consistent seamless printability of NSCs at room was performed to analyze the mRNA expression of
temperature. The preparation of bioink took ~15 min the PN markers on day 3 and day 12 of PN induction
(until loading the bioink cartridge onto the printer) and (Figure 5M). The mRNA expression of PN markers
the printing of each scaffold took ~1 min. The total time PRPH and NEFH was upregulated on day 12 of PN
required to print a 24-well plate was approximately induction compared to day 3. The stemness marker HNK1
~24 min, which is optimal for stem cell bioprinting. was significantly downregulated and the change in the pan
In the bioprinted tissue constructs, the NSCs neural marker TUBB3 was non-significant (Figure 5M).
started to proliferate by 3–5 days post-printing. Cell The mRNA profile (Figure 5M) gives clear clues on the
viability post-printing was confirmed by DAPI staining PN differentiation.
on day 1 (Figure 5A). On day 5, once the cells appeared
settled to grow, PN induction media was added. The 3.4. Cold storage compatibility of the bioprinted
immunofluorescence staining of the cells on different tissues
stages of the induction showed expression of PN marker
NEFH by day 12, indicating the differentiation of the One of the ultimate aims of tissue bioprinting is future
NSCs to PN inside the tissue constructs (Figure 5C), while regenerative medicine applications. This requires short- or
the day 3 sample did not stain for NEFH (Figure 5B). long-term storage of tissues and tissue transportation
By day 12 of neuronal induction, the cells showed clear in ultra-low temperatures. A freeze-thaw study was
morphology of peripheral neuronal fibers, which formed conducted to analyze the storage potential of the bioprinted
a network of PN in the tissue constructs. SEM images of tissues (Figure 6). The ability of the bioprinted and dECM
the bioprinted constructs on day 3 (Figure 5D-F), day 7 scaffold tissues for its cold shock recovery was analyzed
(Figure 5G-I), and day 12 (Figure 5J-L) clearly shows after keeping them frozen for a week in a freezing
International Journal of Bioprinting (2022)–Volume 8, Issue 4 91