Page 48 - IJB-9-1
P. 48
International Journal of Bioprinting Effect of ionic crosslinking on composite membranes
the absorption bands at 3293 and 3120 cm were attributed a relative complicated microenvironment in existence of
−1
to the N-H stretching vibration of amide A and amide B, slight CaCl (0.5 wt%), as illustrated in Figure 6.
2
respectively (Figure 2D). The amide A of the spectrum When a little amount of SFDDS was introduced into the
had direct relationships with changes in collagen triple alginate-based composite bioscaffolds with 0.5 wt% CaCl
helix and hydrogen bonding patterns. The absorption peak during different ionic crosslinking time, weak association
2
at 3293 cm of ADDS3T5 was the amide A band, which between functional groups would be formed, such as weak
−1
is due to N−H stretching vibration and hydrogen bonds. ionic interactions among Ca ions and acidic groups of
2+
When N−H participates in the formation of a hydrogen ALG and weak interaction between ammonium group of
bond, the wavenumber of its stretching vibration would be SFDDS and acidic group of ALG, as shown in Figure 2. Most
shifted to ~3300 cm −1[25-28] . The amide B band was related
to asymmetric stretch vibrations of –NH and =C–H, and A D
+
3
the shift of amide B to higher wavenumber (~3120 cm )
−1
was associated with an increase in free NH–NH 3 groups
+
from both lysine residues and the N-terminus [25-28] . The
same results were observed in ADDS1T5, ADDS2T5, and
ADDS3T5.
3.2. Morphology of alginate-based composite
bioscaffolds with decellularized SFDDS B E
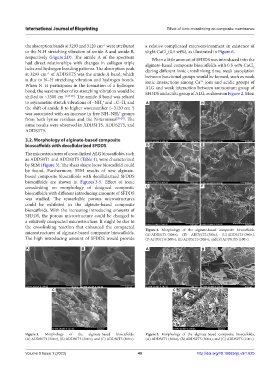
The microstructures of cross-linked ALG bioscaffolds, such
as ADDS0T1 and ADDS0T5 (Table 1), were characterized
by SEM (Figure 3). The sheet shape loose bioscaffold could
be found. Furthermore, SEM results of new alginate-
based composite bioscaffolds with decellularized SFDDS
bioscaffolds are shown in Figures 3-5. Effect of ionic
crosslinking on morphology of designed composite C F
bioscaffolds with different introducing amounts of SFDDS
was studied. The remarkable porous microstructures
could be exhibited in the alginate-based composite
bioscaffolds. With the increasing introducing amounts of
SFDDS, the porous microstructure could be changed to
a relatively compacted microstructure. It might be due to
the crosslinking reaction that enhanced the compacted Figure 4. Morphology of the alginate-based composite bioscaffolds.
microstructures of alginate-based composite bioscaffolds. (A) ADDS1T1 (300×), (B) ADDS1T2 (300×), (C) ADDS1T3 (300×),
The high introducing amount of SFDDS would provide (D ADDS1T4 (300×), (E) ADDS1T5 (300×), and (F) ADDS1T5 (100×).
A B A B
C C
Figure 3. Morphology of the alginate-based bioscaffolds. Figure 5. Morphology of the alginate-based composite bioscaffolds.
(A) ADDS0T1 (300×), (B) ADDS0T5 (300×), and (C) ADDS0T5 (100×). (A) ADDS2T1 (300×), (B) ADDS2T5 (300×), and (C) ADDS2T5 (100×).
Volume 9 Issue 1 (2023) 40 http://doi.org/10.18063/ijb.v9i1.625