Page 194 - IJB-9-2
P. 194
International Journal of Bioprinting A regulated GelMA-MSCs scaffold by three-dimensional bioprinting
As shown in Figure 3D, cells that passed through the 3.7. 3D bioprinting scaffold for cartilage repair
polycarbonate membrane were stained with crystal violet. The GelMA-MSCs bioink was prepared according to the
The number of migrated cells in blank group, negative method above, and a porous cylindrical scaffold with a
control group, and microRNA-410-mimics group was diameter of 5 mm and a height of 5 mm was printed by a
33.75 ± 5.75, 32.25 ± 5.25, and 80.55 ± 6.05, respectively 3D extrusion bioprinter (Figure 5). The printing process is
(Figure 3E). Compared with the negative control group, demonstrated in Videoclip S2.
the number of migrated cells significantly increased in
microRNA-410-mimics group (***, P < 0.001, n = 9). Complete experimental flow chart is shown in
Figure 6A. According to the following three groups, (i)
3.6. Overexpression of microRNA-410 promotes blank group (defect sutured without any treatment); (ii)
proliferation and differentiation of MSCs GelMA-MSCs group (GelMA scaffolds containing only
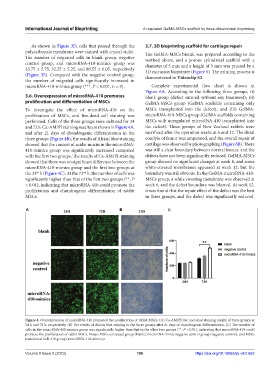
To investigate the effect of microRNA-410 on the MSCs transplanted into the defect); and (iii) GelMA-
proliferation of MSCs, and live-dead cell staining was microRNA-410-MSCs group (GelMA scaffolds containing
performed. Cells of the three groups were cultured for 24 MSCs with upregulated microRNA-410 transplanted into
and 72 h. Ca-AM/PI staining results are shown in Figure 4A, the defect). Three groups of New Zealand rabbits were
and after 21 days of chondrogenic differentiation in the sacrificed after the operation weeks at 6 and 12. The distal
three groups (Figure 4B), the results of Alcian blue staining condyle of femur was amputated, and the overall repair of
showed that the content of acidic mucin in the microRNA- cartilage was observed by photographing (Figure 6B). There
410-mimics group was significantly increased compared was still a clear boundary between normal tissues, and the
with the first two groups. The results of Ca-AM/PI staining defects have not been significantly reduced. GelMA-MSCs
showed that there was no significant difference between the group showed no significant changes at week 6, and some
microRNA-410-mimics group and the first two groups at white-covered membranes appeared at week 12, but the
the 24 h (Figure 4C). At the 72 h, the number of cells was boundary was still obvious. In the GelMA-microRNA-410-
th
nd
significantly higher than that of the first two groups (**, P MSCs group, a white covering membrane was observed at
< 0.01), indicating that microRNA-410 could promote the week 6, and the defect boundary was blurred. At week 12,
proliferation and chondrogenic differentiation of rabbit it was found that the repair effect of the defect was the best
MSCs. in three groups, and the defect was significantly reduced.
A B C
Figure 4. Overexpression of microRNA-410 promoted the proliferation of rabbit MSCs. (A) Ca-AM/PI live and dead staining results of three groups at
24 h and 72 h, respectively. (B) The results of Alcian blue staining in the three groups after 21 days of chondrogenic differentiation. (C) The number of
cells in the microRNA-410-mimics group was significantly higher than that in the other two groups (**, P < 0.01), indicating that microRNA-410 could
promote the proliferation of rabbit MSCs. Notes: MSCs untreated group (blank), microRNA mimic negative control group (negative control), and MSCs
transfected miR-410 group (microRNA-410-mimics).
Volume 9 Issue 2 (2023) 186 https://doi.org/10.18063/ijb.v9i2.662