Page 305 - IJB-9-2
P. 305
International Journal of Bioprinting Zn-doped coatings with osteogenic and antibacterial properties
A B C
D E F
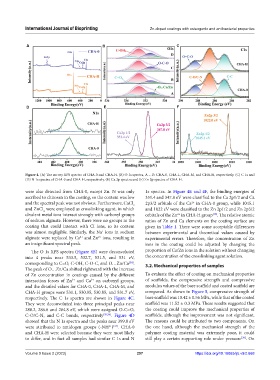
Figure 4. (A) The survey XPS spectra of CHA-0 and CHA-H. (B) O 1s spectra, A – D: CHA-0, CHA-L, CHA-M, and CHA-H, respectively. (C) C 1s and
(D) N 1s spectra of CHA-0 and CHA-H, respectively. (E) Ca 2p spectra and (F) Zn 2p spectra of CHA-H.
were also detected from CHA-0, except Zn. N was only 1s spectra. In Figure 4E and 4F, the binding energies of
ascribed to chitosan in the coating, so the content was low 351.4 and 347.8 eV were classified to the Ca 2p1/2 and Ca
and the spectral peak was not obvious. Furthermore, CaCl 2p3/2 orbitals of the Ca in CHA-0 group, while 1045.1
2+
2
and ZnCl were employed as crosslinking agent, in which and 1022 eV were classified to the Zn 2p1/2 and Zn 2p3/2
2
divalent metal ions interact strongly with carboxyl groups orbitals of the Zn in CHA-H group . The relative atomic
2+
[38]
of sodium alginate. However, there were no groups in the ratios of Zn and Ca elements on the coating surface are
coating that could interact with Cl ions, so its content given in Table 1. There were some acceptable differences
-
was almost negligible. Similarly, the Na ions in sodium between experimental and theoretical values caused by
+
alginate were replaced by Ca and Zn ions, resulting in experimental errors. Therefore, the concentration of Zn
2+
2+
an insignificant spectral peak. ions in the coating could be adjusted by changing the
The O 1s XPS spectra (Figure 4B) were deconvoluted proportion of Ca/Zn ions in the solution without changing
into 4 peaks near 533.5, 532.7, 531.5, and 531 eV, the concentration of the crosslinking agent solution.
corresponding to C=O, C-OH, C-O-C, and O…Zn/Ca . 3.2. Mechanical properties of samples
[34]
The peak of O…Zn/Ca shifted rightward with the increase
of Zn concentration in coatings caused by the different To evaluate the effect of coating on mechanical properties
interaction forces of Zn and Ca on carboxyl groups, of scaffolds, the compressive strength and compressive
2+
2+
and the detailed values for CHA-0, CHA-L, CHA-M, and modulus values of the bare scaffold and coated scaffold are
CHA-H groups were 531.1, 530.95, 530.85, and 531.7 eV, compared. As shown in Figure 5, compressive strength of
respectively. The C 1s spectra are shown in Figure 4C. bare scaffold was 10.42 ± 0.56 MPa, while that of the coated
They were deconvoluted into three principal peaks near scaffold was 11.52 ± 0.3 MPa. These results suggested that
288.2, 286.6 and 284.8 eV, which were assigned O-C=O, the coating could improve the mechanical properties of
C-O/C-N, and C-C bonds, respectively [35,36] . Figure 4D scaffolds, although the improvement was not significant.
showed that the N 1s spectra and the peaks near 399.8 eV The reasons could be attributed to two components. On
were attributed to amidogen groups (-NH ) . CHA-0 the one hand, although the mechanical strength of the
3+ [37]
and CHA-H were selected because they were most likely polymer coating material was extremely poor, it could
to differ, and in fact all samples had similar C 1s and N still play a certain supporting role under pressure . On
[39]
Volume 9 Issue 2 (2023) 297 https://doi.org/10.18063/ijb.v9i2.668