Page 306 - IJB-9-2
P. 306
International Journal of Bioprinting Zn-doped coatings with osteogenic and antibacterial properties
the other hand, it was inevitable that some defects would large to be interfered by the elements of the sample itself.
appear on the scaffold surface during the preparation In particular, it was interesting to note that the Ca/P ratio
process. The addition of the adhesive coating properly of CHA-H was higher than that of CHA-0, even though
filled these defects, thus avoiding the premature collapse the calcium content in the coating of CHA-H group was
of scaffolds. lower than that of CHA-0 group. It indicated that the
higher the Zn content in the coating, the higher the Ca/P
3.3. Degradation properties of samples ratio in the induced apatite deposition layer, which may
Numerous studies have shown that HA scaffolds could be attributed to the complex nucleation mechanism of
induce apatite deposition on the surface in SBF, which was apatite . Moreover, the surface morphology of CHA-0
[43]
related to the bioactivity properties of scaffolds [40,41] . The and CHA-H samples also indicated that the coating with
HA, CHA-0, and CHA-H scaffolds were soaked in the SBF higher Zn concentration might have a stronger ability to
for 14 days at 37°C, and then the surface morphologies induce apatite deposition.
were observed by SEM. As showed in Figure 6A, coralloid To evaluate the effect of coatings on the degradation of
deposits were formed on the surface of the HA scaffold. scaffolds, the bare HA scaffold and the CHA-M scaffolds
The EDS results (Figure 6B) confirmed that the deposits with different number of coating layers (3, 6, and 9 layers,
were calcium phosphate, and the calcium to phosphorus respectively) were immersed in Tris-HCl for 7, 14, 21, and
ratio was 1.37. Studies have suggested that calcium
phosphate could be classified as HA when the Ca/P ratio 28 days. The degradation curves are shown in Figure 7A,
was between 1.3 and 2.0 . As for the scaffold with coating The HA scaffold had the highest mass loss of 2.93% among
[42]
(Figure 6C–F), the apatite deposits on the surface were all groups after 4 weeks. For coated scaffolds, the mass
less and appeared as spherical clusters, indicating that the loss rate showed a dependence on the number of coating
addition of coating inhibited the deposition of apatite to a layers. As the number of coating layers increased, the
certain extent. In addition, the EDS results showed that the weight loss rate of scaffolds was slower, showing a delayed
Ca/P ratio of the coated scaffold was significantly higher effect. Consequently, the addition of coating inhibited the
than that of the HA scaffold, which can be attributed to the degradation of scaffolds.
fact that the penetration depth of EDS detection was too Figure 7B showed the variation of pH values of Tris-
HCl solution after soaking the samples. During the whole
Table 1. Planned and experimentally obtained molar ratios soaking process, except for the CHA-M (9), the pH value
of Ca:Zn in coatings. of the other groups decreased first and then tended to
be stable, but the reasons were not exactly the same. For
Sample Planned (in the crosslinking XPS elemental
agent solution) analysis (in samples) the HA scaffold, its degradation products were weakly
Mass ratio of Molar ratio of Molar ratio alkaline, while the deposition of apatite would consume the
CaCl :ZnCl 2 Ca +:Zn + of Ca:Zn hydroxide ions in the solution, leading to a decrease of pH
2
2
2
CHA-0 0:1 0:1 0:1 value [44,45] . Consequently, the pH value of the immersion
CHA-H 1:1 1:1.23 1:1.31 solution showed a state of fluctuation. As for the coated
scaffolds, in addition to the effect caused by apatite
CHA-M 1:3 1:3.68 1:3.48 deposition, the decrease of the pH value of the solution
CHA-L 1:7 1:8.58 1:6.73 might be due to the trace residue of acetic acid introduced
A B
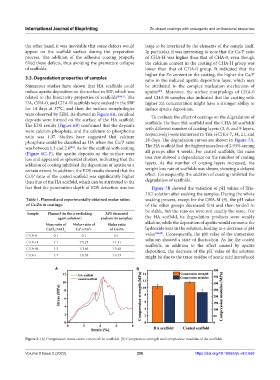
Figure 5. (A) Compression stress-strain curves of the scaffolds. (B) Compressive strength and compressive modulus of the scaffolds.
Volume 9 Issue 2 (2023) 298 https://doi.org/10.18063/ijb.v9i2.668