Page 395 - IJB-9-2
P. 395
International Journal of Bioprinting In situ 3D bioprinter for skin wound healing
A B C D
E F G H
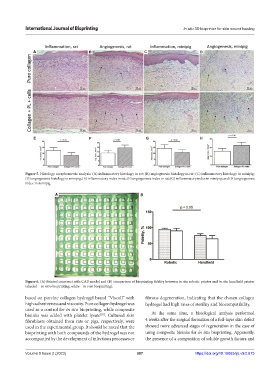
Figure 5. Histology morphometric analysis: (A) inflammatory histology in rat; (B) angiogenesis histology in rat; (C) inflammatory histology in minipig;
(D) angiogenesis histology in minipig; (E) inflammatory index in rat; (F) angiogenesis index in rat; (G) inflammatory index in minipig; and (H) angiogenesis
index in minipig.
A B
Figure 6. (A) Printed construct with CAD model and (B) comparison of bioprinting fidelity between in situ robotic printer and in situ handheld printer
(shaded – in vitro bioprinting, white – in vivo bioprinting).
based on porcine collagen hydrogel brand “Viscoll” with fibrous degeneration, indicating that the chosen collagen
high adhesiveness and viscosity. Pure collagen hydrogel was hydrogel had high rates of sterility and biocompatibility.
used as a control for in situ bioprinting, while composite
[33]
bioinks was added with platelet lysate . Cultured skin At the same time, a histological analysis performed
fibroblasts obtained from rats or pigs, respectively, were 4 weeks after the surgical formation of a full-layer skin defect
used in the experimental group. It should be noted that the showed more advanced stages of regeneration in the case of
bioprinting with both compounds of the hydrogel was not using composite bioinks for in situ bioprinting. Apparently,
accompanied by the development of infectious processes or the presence of a composition of soluble growth factors and
Volume 9 Issue 2 (2023) 387 https://doi.org/10.18063/ijb.v9i2.675