Page 396 - IJB-9-2
P. 396
International Journal of Bioprinting In situ 3D bioprinter for skin wound healing
A B
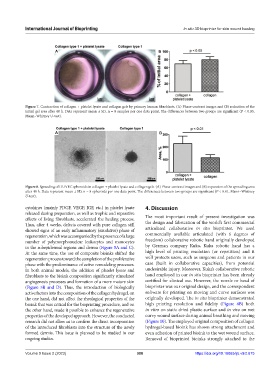
Figure 7. Contraction of collagen + platelet lysate and collagen gels by primary human fibroblasts. (A) Phase-contrast images and (B) reduction of the
initial gel area after 48 h. Data represent mean ± SD, n = 8 samples per one data point. The differences between two groups are significant (P < 0.05,
Mann–Whitney U-test).
A B
Figure 8. Spreading of HUVEC spheroids in collagen + platelet lysate and collagen gels. (A) Phase-contrast images and (B) expansion of the spreading area
after 48 h. Data represent mean ± SD, n = 8 spheroids per one data point. The differences between two groups are significant (P < 0.01, Mann–Whitney
U-test).
cytokines (mainly PDGF, VEGF, IGF, etc.) in platelet lysate 4. Discussion
released during preparation, as well as trophic and reparative
effects of living fibroblasts, accelerated the healing process. The most important result of present investigation was
Thus, after 4 weeks, defects covered with pure collagen still the design and fabrication of the world’s first commercial
showed signs of an early inflammatory (exudative) phase of articulated collaborative in situ bioprinter. We used
regeneration, which was accompanied by the presence of a large commercially available articulated (with 6 degrees of
number of polymorphonuclear leukocytes and monocytes freedom) collaborative robotic hand originally developed
in the subepidermal regions and derma (Figure 5A and C). by German company Kuka. Kuka robotic hand has a
At the same time, the use of composite bioinks shifted the high level of printing resolution (or repetition) and it
regeneration process toward the completion of the proliferative well protects users, such as surgeons and patients in our
phase with the predominance of active remodeling processes. case (built in collaborative capacities), from potential
In both animal models, the addition of platelet lysate and undesirable injury. Moreover, Kuka’s collaborative robotic
fibroblasts to the bioink composition significantly stimulated hand employed in our in situ bioprinter has been already
angiogenesis processes and formation of a more mature skin certified for clinical use. However, the nozzle or head of
(Figure 5B and D). Thus, the introduction of biologically bioprinter was our original design, and the correspondent
active factors into the composition of the collagen hydrogel, on software for printing on moving and curve surfaces was
the one hand, did not affect the rheological properties of the originally developed. The in situ bioprinter demonstrated
bioink that was critical for the bioprinting procedure, and on high printing resolution and fidelity (Figure 6B) both
the other hand, made it possible to enhance the regenerative in vitro on static dried plastic surface and in vivo on wet
properties of the developed approach. However, the conducted curvy wound surface during animal breathing and moving
research did not allow us to confirm the direct incorporation (Figure 10). The employed original composition of collagen
of the introduced fibroblasts into the structure of the newly hydrogel-based bioink has shown strong attachment and
formed dermis. This issue is planned to be studied in our even adhesion of printed bioink to the wet wound surface.
ongoing studies. Removal of bioprinted bioinks strongly attached to the
Volume 9 Issue 2 (2023) 388 https://doi.org/10.18063/ijb.v9i2.675