Page 369 - IJB-9-5
P. 369
International Journal of Bioprinting Multifunctional hydrogel surgical training model
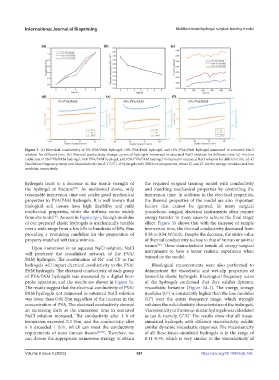
Figure 3. (a) Electrical conductivity of 5% PVA/PAM hydrogel, 10% PVA/PAM hydrogel, and 15% PVA/PAM hydrogel immersed in saturated NaCl
solution for different time. (b) Thermal conductivity change curves of hydrogels immersed in saturated NaCl solution for different time. (c) Friction
coefficient of 5% PVA/PAM hydrogel, 10% PVA/PAM hydrogel, and 15% PVA/PAM hydrogel immersed in saturated NaCl solution for different time. (d–f)
Oscillation frequency sweep and viscoelasticity (tan δ, G″/G′) of hydrogels with different components, where G′ and G″ are the storage modulus and loss
modulus, respectively.
hydrogels leads to a decrease in the tensile strength of the required surgical training model with conductivity
the hydrogel at fracture . As mentioned above, only and matching mechanical properties by controlling the
[25]
reasonable immersion time can confer good mechanical immersion time. In addition to the electrical properties,
properties to PVA/PAM hydrogels. It is well known that the thermal properties of the model are also important
biological soft tissues have high flexibility and mild factors that cannot be ignored. In many surgical
mechanical properties, while the stiffness varies widely procedures, surgical electrical instruments often require
from site to site . As seen in Figure 2g–i, Young’s modulus energy transfer in many cases to achieve the final target
[26]
of our prepared elastic hydrogels is mechanically tunable effect. Figure 3b shows that with the increase of sample
over a wide range from a few kPa to hundreds of kPa, thus immersion time, the thermal conductivity decreased from
providing a promising candidate for the preparation of 0.58 to 0.54 W/m/K. Despite the decrease, the entire value
property-matched soft tissue mimics. of thermal conductivity is close to that of human or animal
tissues . These characteristics benefit all energy surgical
[29]
Upon immersion in an aqueous NaCl solution, NaCl
will penetrate the crosslinked network of the PVA/ instruments to have a better realistic experience when
trained on the model.
PAM hydrogels. The combination of Na and Cl in the
-
+
hydrogels will impart electrical conductivity to the PVA/ Rheological measurements were also performed to
PAM hydrogels. The electrical conductivity of each group demonstrate the viscoelastic and wet-slip properties of
of PVA/PAM hydrogels was measured by a digital four- tissue-like elastic hydrogels. Rheological frequency scans
probe apparatus, and the results are shown in Figure 3a. of the hydrogels confirmed that they exhibit dynamic
The results suggest that the electrical conductivity of PVA/ viscoelastic behavior (Figure 3d–f). The energy storage
PAM hydrogels not immersed in saturated NaCl solution modulus (G′) is consistently higher than the loss modulus
was lower than 0.01 S/m regardless of the increase in the (G″) over the entire frequency range, which strongly
concentration of PVA. The electrical conductivity showed validates the solid elasticity characteristics of the hydrogels.
an increasing form as the immersion time in saturated Viscoelasticity of the tissue-mimic hydrogels was calculated
NaCl solution increased. The conductivity after 1 h of as tan δ, namely, G″/G′ The results show that all tissue-
immersion exceeded 0.2 S/m, and the conductivity after simulated hydrogels with different viscoelasticity exhibit
6 h exceeded 1 S/m, which can meet the conductivity similar dynamic viscoelastic responses. The viscoelasticity
requirements of most human tissues [27,28] . Therefore, we of all these tissue-simulated hydrogels is in the range of
can choose the appropriate immersion strategy to obtain 0.11–0.55, which is very similar to the viscoelasticity of
Volume 9 Issue 5 (2023) 361 https://doi.org/10.18063/ijb.766