Page 404 - IJB-9-5
P. 404
International Journal of Bioprinting A sturgeon cartilage extracellular matrix-derived bioactive bioink
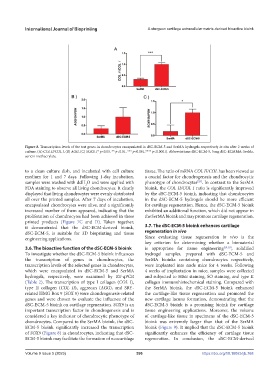
Figure 8. Transcription levels of the test genes in chondrocytes encapsulated in dSC-ECM-5 and SerMA hydrogels respectively in situ after 2 weeks of
culture. (A) COL II/COL I, (B) AGG, (C) SOX9. (* p<0.05, ** p<0.01, *** p<0.001,**** p<0.0001). Abbreviations: dSC-ECM-5, 5 mg dSC-ECMMA; SerMa,
sericin methacrylate.
to a clean culture dish, and incubated with cell culture tissue. The ratio of mRNA COL II/COL has been viewed as
medium for 1 and 7 days. Following 1-day incubation, a crucial factor for chondrogenesis and the chondrocytic
samples were washed with ddH O and were applied with phenotype of chondrocytes . In contrast to the SerMA
[28]
2
FDA staining to observe all living chondrocytes. It clearly bioink, the COL II/COL I ratio is significantly improved
displayed that living chondrocytes were evenly distributed by the dSC-ECM-5 bioink, indicating that chondrocytes
all over the printed samples. After 7 days of incubation, in the dSC-ECM-5 hydrogels should be more efficient
encapsulated chondrocytes were alive, and a significantly for cartilage regeneration. Hence, the dSC-ECM-5 bioink
increased number of them appeared, indicating that the exhibited an additional function, which did not appear in
proliferation of chondrocytes had been achieved in these the SerMA bioink and may promote cartilage regeneration.
printed products (Figure 7C and D). Taken together,
it demonstrated that the dSC-ECM-derived bioink, 3.7. The dSC-ECM-5 bioink enhances cartilage
dSC-ECM-5, is suitable for 3D bioprinting and tissue regeneration in vivo
engineering applications. Since evaluating tissue regeneration in vivo is the
key criterion for determining whether a biomaterial
3.6. The bioactive function of the dSC-ECM-5 bioink is appropriate for tissue engineering [29,30] , solidified
To investigate whether the dSC-ECM-5 bioink influences hydrogel samples, prepared with dSC-ECM-5 and
the transcription of genes in chondrocytes, the SerMA bioinks containing chondrocytes respectively,
transcription levels of the selected genes in chondrocytes, were implanted into nude mice for 4 weeks. Following
which were encapsulated in dSC-ECM-5 and SerMA 4 weeks of implantation in mice, samples were collected
hydrogels, respectively, were examined by RT-qPCR and subjected to H&E staining, SO staining, and type II
(Table 2). The transcription of type I collagen (COL I), collagen immunohistochemical staining. Compared with
type II collagen (COL II), aggrecan (AGG), and SRY‐ the SerMA bioink, the dSC-ECM-5 bioink enhanced
related HMG Box 9 (SOX 9) were chondrogenesis-related the cartilage-like tissue regeneration and promoted the
genes and were chosen to evaluate the influence of the new cartilage lacuna formation, demonstrating that the
dSC-ECM-5 bioink on cartilage regeneration. SOX9 is an dSC-ECM-5 bioink is a promising bioink for cartilage
important transcription factor in chondrogenesis and is tissue engineering applications. Moreover, the volume
considered a key indicator of chondrocytic phenotype of of cartilage-like tissue in specimens of the dSC-ECM-5
chondrocytes. Compared to the SerMA bioink, the dSC- bioink was extremely larger than that of the SerMA
ECM-5 bioink significantly increased the transcription bioink (Figure 9). It implied that the dSC-ECM-5 bioink
of SOX9 (Figure 8) in chondrocytes, indicating that dSC- significantly enhances the efficiency of cartilage tissue
ECM-5 bioink may facilitate the formation of neocartilage regeneration. In conclusion, the dSC-ECM-derived
Volume 9 Issue 5 (2023) 396 https://doi.org/10.18063/ijb.768