Page 447 - IJB-9-6
P. 447
International Journal of Bioprinting 3D bioprinting for lung tissue
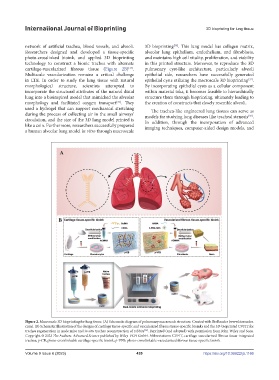
network of artificial trachea, blood vessels, and alveoli. 3D bioprinting . This lung model has collagen matrix,
[36]
Researchers designed and developed a tissue-specific alveolar lung epithelium, endothelium, and fibroblasts,
photo-crosslinked bioink, and applied 3D bioprinting and maintains high cell vitality, proliferation, and viability
technology to construct a bionic trachea with alternate in this printed structure. Moreover, to reproduce the 3D
cartilage-vascularized fibrous tissue (Figure 2B) . pulmonary cyst-like architecture, particularly alveoli
[34]
Multiscale vascularization remains a critical challenge epithelial side, researchers have successfully generated
in LTE. In order to study the lung tissue with natural epithelial cysts utilizing the macroscale 3D bioprinting .
[37]
morphological structure, scientists attempted to By incorporating epithelial cysts as a cellular component
incorporate the structural attributes of the natural distal within material inks, it becomes feasible to hierarchically
lung into a bioinspired model that mimicked the alveolar structure them through bioprinting, ultimately leading to
morphology and facilitated oxygen transport . They the creation of constructs that closely resemble alveoli.
[35]
used a hydrogel that can support mechanical stretching The trachea-like engineered lung tissues can serve as
during the process of collecting air in the small airways’ models for studying lung diseases like tracheal stenosis .
[38]
circulation, and the size of the 3D lung model printed is In addition, through the incorporation of advanced
like a coin. Furthermore, researchers successfully prepared imaging techniques, computer-aided design models, and
a human alveolar lung model in vitro through macroscale
Figure 2. Macroscale 3D bioprinting for lung tissue. (A) Schematic diagram of pulmonary macroscale structure. Created with BioRender (www.biorender.
com). (B) Schematic illustration of the designs of cartilage tissue‐specific and vascularized fibrous tissue‐specific bioinks and the 3D‐bioprinted CVFIT for
trachea regeneration in nude mice and in situ trachea reconstruction of rabbits . Reprinted (and adapted) with permission from John Wiley and Sons.
[34]
Copyright © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH. Abbreviations: CVFIT, cartilage‐vascularized fibrous tissue‐integrated
trachea; p‐CB, photo-crosslinkable cartilage‐specific bioink; p‐VFB: photo-crosslinkable-vascularized fibrous tissue‐specific bioink.
Volume 9 Issue 6 (2023) 439 https://doi.org/10.36922/ijb.1166