Page 311 - v11i4
P. 311
International Journal of Bioprinting 3D scaffold prevents tendon ossification
and must deform to accommodate mechanical loads. Our demonstrated that, compared to SF scaffolds, SF–HPC
findings suggest that the SF–HPC scaffolds possess optimal scaffolds maintained a well-preserved elastic modulus
toughness, ductility, and elastic modulus to withstand the after multiple cycles of tensile loading, indicating favorable
tensile forces required for functional Achilles tendon repair. resistance to stress fatigue (Figure 3C and D).
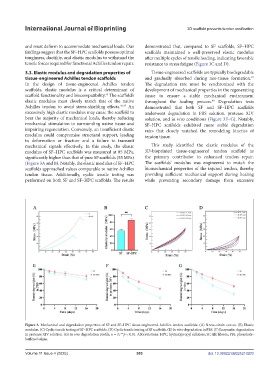
3.3. Elastic modulus and degradation properties of Tissue-engineered scaffolds are typically biodegradable
48
tissue-engineered Achilles tendon scaffolds and gradually absorbed during neo-tissue formation.
In the design of tissue-engineered Achilles tendon The degradation rate must be synchronized with the
scaffolds, elastic modulus is a critical determinant of development of mechanical properties in the regenerating
scaffold functionality and biocompatibility. The scaffold’s tissue to ensure a stable mechanical environment
45
elastic modulus must closely match that of the native throughout the healing process. Degradation tests
49
Achilles tendon to avoid stress-shielding effects. 46,47 An demonstrated that both SF and SF–HPC scaffolds
excessively high elastic modulus may cause the scaffold to underwent degradation in PBS solution, protease XIV
bear the majority of mechanical loads, thereby reducing solution, and in vivo conditions (Figure 3E–G). Notably,
mechanical stimulation to surrounding native tissue and SF–HPC scaffolds exhibited more stable degradation
impairing regeneration. Conversely, an insufficient elastic rates that closely matched the remodeling kinetics of
modulus could compromise structural support, leading tendon tissue.
to deformation or fracture and a failure to transmit
mechanical signals effectively. In this study, the elastic This study identified the elastic modulus of the
modulus of SF–HPC scaffolds was measured at 85 MPa, 3D-bioprinted tissue-engineered tendon scaffold as
significantly higher than that of pure SF scaffolds (55 MPa) the primary contributor to enhanced tendon repair.
(Figure 3A and B). Notably, the elastic modulus of SF–HPC The scaffolds’ modulus was engineered to match the
scaffolds approached values comparable to native Achilles biomechanical properties of the injured tendon, thereby
tendon tissue. Additionally, cyclic tensile testing was providing sufficient mechanical support during healing
performed on both SF and SF–HPC scaffolds. The results while preventing secondary damage from excessive
Figure 3. Mechanical and degradation properties of SF and SF–HPC tissue-engineered Achilles tendon scaffolds. (A) Stress–strain curves. (B) Elastic
modulus. (C) Cyclic tensile testing of SF–HPC scaffolds. (D) Cyclic tensile testing of SF scaffolds. (E) In vitro degradation in PBS. (F) Enzymatic degradation
in protease XIV solution. (G) In vivo degradation profile. n = 3; **p < 0.01. Abbreviations: HPC, hydroxypropyl cellulose; SF, silk fibroin; PBs, phosphate-
buffered saline.
Volume 11 Issue 4 (2025) 303 doi: 10.36922/IJB025210203