Page 313 - v11i4
P. 313
International Journal of Bioprinting 3D scaffold prevents tendon ossification
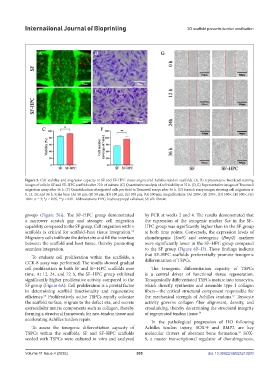
Figure 5. Cell viability and migration capacity in SF and SF–HPC tissue-engineered Achilles tendon scaffolds. (A, B) representative live/dead staining
images of cells in SF and SF–HPC scaffolds after 72 h of culture. (C) Quantitative analysis of cell viability at 72 h. (D, E) Representative images of Transwell
migration assay after 36 h. (F) Quantification of migrated cells per field in Transwell assays after 36 h. (G) Scratch assay images showing cell migration at
0, 12, 24, and 36 h. Scale bars: (A) 50 μm, (B) 50 μm, (D) 100 μm, (E) 100 μm, (G) 500 μm; magnifications: (A) 200×, (B) 200×, (D) 500×, (E) 500×, (G)
100×. n = 3; *p < 0.05, **p < 0.01. Abbreviations: HPC, hydroxypropyl cellulose; SF, silk fibroin.
groups (Figure 5G). The SF–HPC group demonstrated by PCR at weeks 2 and 4. The results demonstrated that
a narrower scratch gap and stronger cell migration the expression of the tenogenic marker Scx in the SF–
capability compared to the SF group. Cell migration within HPC group was significantly higher than in the SF group
scaffolds is critical for scaffold–host tissue integration. at both time points. Conversely, the expression levels of
55
Migratory cells infiltrate the defect site and fill the interface chondrogenic (Sox9) and osteogenic (Bmp2) markers
between the scaffold and host tissue, thereby promoting were significantly lower in the SF–HPC group compared
seamless integration. to the SF group (Figure 6B–D). These findings indicate
that SF–HPC scaffolds preferentially promote tenogenic
To evaluate cell proliferation within the scaffolds, a
CCK-8 assay was performed. The results showed gradual differentiation of TSPCs.
cell proliferation in both SF and SF–HPC scaffolds over The tenogenic differentiation capacity of TSPCs
time. At 12, 24, and 72 h, the SF–HPC group exhibited is a central driver of functional tissue regeneration.
significantly higher proliferative activity compared to the Tenogenically differentiated TSPCs mature into tenocytes,
SF group (Figure 6A). Cell proliferation is a pivotal factor which directly synthesize and assemble type I collagen
in determining scaffold functionality and regenerative fibers—the critical structural component responsible for
efficiency. Proliferatively active TSPCs rapidly colonize the mechanical strength of Achilles tendons. Tenocyte
57
56
the scaffold surface, migrate to the defect site, and secrete activity governs collagen fiber alignment, density, and
extracellular matrix components such as collagen, thereby crosslinking, thereby determining the structural integrity
forming a structural framework for neo-tendon tissue and of regenerated tendon tissue. 58
accelerating Achilles tendon repair. In the pathological progression of HO following
To assess the tenogenic differentiation capacity of Achilles tendon injury, SOX-9 and BMP2 are key
TSPCs within the scaffolds, SF and SF–HPC scaffolds molecular drivers of aberrant bone formation. SOX-
59
seeded with TSPCs were cultured in vitro and analyzed 9, a master transcriptional regulator of chondrogenesis,
Volume 11 Issue 4 (2025) 305 doi: 10.36922/IJB025210203