Page 314 - v11i4
P. 314
International Journal of Bioprinting 3D scaffold prevents tendon ossification
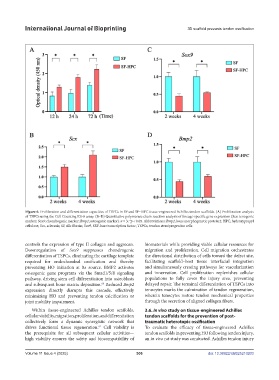
Figure 6. Proliferation and differentiation capacities of TSPCs in SF and SF–HPC tissue-engineered Achilles tendon scaffolds. (A) Proliferation analysis
of TSPCs using the Cell Counting Kit-8 assay. (B–D) Quantitative polymerase chain reaction analysis of lineage-specific gene expression (Scx: tenogenic
marker; Sox9: chondrogenic marker; Bmp2; osteogenic marker). n = 3; *p < 0.05. Abbreviations: Bmp2, bone morphogenetic protein 2; HPC, hydroxypropyl
cellulose; Scx, scleraxis; SF, silk fibroin; Sox9, SRY-box transcription factor; TSPCs, tendon stem/progenitor cells.
controls the expression of type II collagen and aggrecan. biomaterials while providing viable cellular resources for
Downregulation of Sox9 suppresses chondrogenic migration and proliferation. Cell migration orchestrates
differentiation of TSPCs, eliminating the cartilage template the directional distribution of cells toward the defect site,
required for endochondral ossification and thereby facilitating scaffold–host tissue interfacial integration
preventing HO initiation at its source. BMP2 activates and simultaneously creating pathways for vascularization
osteogenic gene programs via the Smad1/5/8 signaling and innervation. Cell proliferation replenishes cellular
pathway, driving stem cell differentiation into osteoblasts populations to fully cover the injury area, preventing
and subsequent bone matrix deposition. Reduced Bmp2 delayed repair. The terminal differentiation of TSPCs into
60
expression directly disrupts this cascade, effectively tenocytes marks the culmination of tendon regeneration,
minimizing HO and preventing tendon calcification or wherein tenocytes restore tendon mechanical properties
joint mobility impairment. through the secretion of aligned collagen fibers.
Within tissue-engineered Achilles tendon scaffolds, 3.6. In vivo study on tissue-engineered Achilles
cellular viability, migration, proliferation, and differentiation tendon scaffolds for the prevention of post-
collectively form a dynamic synergistic network that traumatic heterotopic ossification
drives functional tissue regeneration. Cell viability is To evaluate the efficacy of tissue-engineered Achilles
61
the prerequisite for all subsequent cellular activities— tendon scaffolds in preventing HO following tendon injury,
high viability ensures the safety and biocompatibility of an in vivo rat study was conducted. Achilles tendon injury
Volume 11 Issue 4 (2025) 306 doi: 10.36922/IJB025210203