Page 312 - v11i4
P. 312
International Journal of Bioprinting 3D scaffold prevents tendon ossification
stress concentration. Furthermore, as supported by migration. High porosity enhances cell infiltration and
52
prior research, an optimal elastic modulus maintains nutrient diffusion, thereby promoting cellular activities
physiological tendon function and significantly enhances within the scaffold. 53
the proliferation of tendon-derived cells on the scaffolds,
ultimately facilitating superior tendon regeneration. 50 3.5. Cell viability, migration, proliferation, and
differentiation in tissue-engineered Achilles
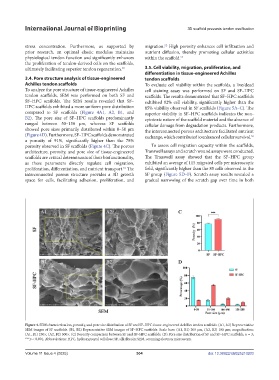
3.4. Pore structure analysis of tissue-engineered tendon scaffolds
Achilles tendon scaffolds To evaluate cell viability within the scaffolds, a live/dead
To analyze the pore structure of tissue-engineered Achilles cell staining assay was performed on SF and SF–HPC
tendon scaffolds, SEM was performed on both SF and scaffolds. The results demonstrated that SF–HPC scaffolds
SF–HPC scaffolds. The SEM results revealed that SF– exhibited 92% cell viability, significantly higher than the
HPC scaffolds exhibited a more uniform pore distribution 85% viability observed in SF scaffolds (Figure 5A–C). The
compared to SF scaffolds (Figure 4A1, A2, B1, and superior viability in SF–HPC scaffolds indicates the non-
B2). The pore size of SF–HPC scaffolds predominantly cytotoxic nature of the scaffold material and the absence of
ranged between 50–150 μm, whereas SF scaffolds cellular damage from degradation products. Furthermore,
showed pore sizes primarily distributed within 0–50 μm the interconnected porous architecture facilitated nutrient
(Figure 4D). Furthermore, SF–HPC scaffolds demonstrated exchange, which contributed to enhanced cellular survival. 54
a porosity of 91%, significantly higher than the 78%
porosity observed in SF scaffolds (Figure 4C). The porous To assess cell migration capacity within the scaffolds,
architecture, porosity, and pore size of tissue-engineered Transwell assays and scratch wound assays were conducted.
scaffolds are critical determinants of their biofunctionality, The Transwell assay showed that the SF–HPC group
as these parameters directly regulate cell migration, exhibited an average of 115 migrated cells per microscopic
proliferation, differentiation, and nutrient transport. The field, significantly higher than the 85 cells observed in the
51
interconnected porous structure provides a 3D growth SF group (Figure 5D–F). Scratch assay results revealed a
space for cells, facilitating adhesion, proliferation, and gradual narrowing of the scratch gap over time in both
Figure 4. SEM characterization, porosity, and pore size distribution of SF and SF–HPC tissue-engineered Achilles tendon scaffolds. (A1, A2) Representative
SEM images of SF scaffolds. (B1, B2) Representative SEM images of SF–HPC scaffolds. Scale bars: (A1, B1) 200 μm, (A2, B2) 100 μm; magnification:
(A1, B1) 250×, (A2, B2) 500×. (C) Porosity comparison between SF and SF–HPC scaffolds. (D) Pore size distribution of SF and SF–HPC scaffolds. n = 3;
***p < 0.001. Abbreviations: HPC, hydroxypropyl cellulose; SF, silk fibroin; SEM, scanning electron microscopy.
Volume 11 Issue 4 (2025) 304 doi: 10.36922/IJB025210203