Page 73 - ITPS-7-1
P. 73
INNOSC Theranostics and
Pharmacological Sciences Docking study of quinoline-3-carbaldehyde derives
interactions can lead to toxic or adverse effects due to clinically utilized drugs, involving the addition or removal
decreased drug clearance or the accumulation of drugs or of specific functional groups through hydroxylation,
their metabolites. Table 6 reveals that the lead compounds demethylation, and dealkylation processes .
[27]
act as inhibitors of CYP2C19 while serving as substrates
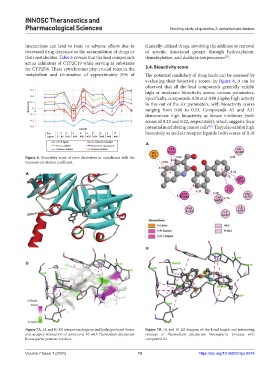
for CYP2D6. These cytochromes play crucial roles in the 3.4. Bioactivity score
metabolism and elimination of approximately 25% of The potential candidacy of drug leads can be assessed by
evaluating their bioactivity scores. In Figure 6, it can be
observed that all the lead compounds generally exhibit
0.3
high or moderate bioactivity across various parameters.
0.2
Specifically, compounds A20 and A48 display high activity
0.1
Bioactivity 0 in five out of the six parameters, with bioactivity scores
ranging from 0.00 to 0.33. Compounds A5 and A31
-0.1 0 1 2 3 4 5 6 7 8 9 10 demonstrate high bioactivity as kinase inhibitors (with
-0.2 scores of 0.23 and 0.22, respectively), which suggests their
[28]
-0.3 potential in inhibiting cancer cells . They also exhibit high
Ligands
Key 1 2 3 4 5 6 7 8 9 10 bioactivity as nuclear receptor ligands (with scores of 0.18
Ligand 5 A5 A31 A36 A20 A33 A34 A45 A48 A49
GPCR ligand Ion channel modulator A
Kinase inhibitor Nuclear receptor ligand
Protease inhibitor Enzyme inhibitor
Figure 6. Bioactivity score of pure derivatives in compliance with the
Pearson’s correlation coefficient.
A
B
B
Figure 7A. (A and B) 3D interaction diagram and hydrogen bond donor Figure 7B. (A and B) 2D diagram of the bond length and interacting
and acceptor interaction of compound A5 with Plasmodium falciparum residues of Plasmodium falciparum histoaspartic protease with
histoaspartic protease residues. compound A5.
Volume 7 Issue 1 (2024) 10 https://doi.org/10.36922/itps.0976