Page 72 - ITPS-7-1
P. 72
INNOSC Theranostics and
Pharmacological Sciences Docking study of quinoline-3-carbaldehyde derives
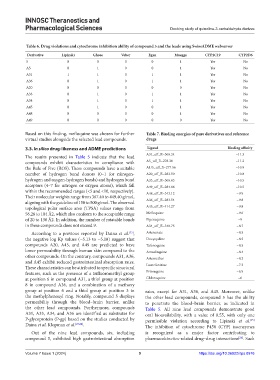
Table 6. Drug violations and cytochrome inhibition ability of compound 5 and the leads using SwissADME webserver
Derivative Lipinski Ghose Veber Egan Muegge CYP2C19 CYP2D6
5 0 0 0 0 1 Yes No
A5 0 1 0 0 1 Yes No
A31 1 1 0 1 1 Yes No
A36 0 1 0 1 1 Yes No
A20 0 0 0 0 0 Yes No
A33 0 1 0 1 1 Yes No
A34 0 1 0 1 1 Yes No
A45 0 1 0 0 1 Yes No
A48 0 0 0 0 1 Yes No
A49 0 0 0 0 0 Yes No
Based on this finding, mefloquine was chosen for further Table 7. Binding energies of pure derivatives and reference
virtual studies alongside the selected lead compounds. drugs
3.3. In silico drug-likeness and ADME predictions Ligand Binding affinity
The results presented in Table 5 indicate that the lead A31_uff_E=300.34 −11.3
compounds exhibit characteristics in compliance with A5_uff_E=278.30 −11.2
the Rule of Five (RO5). These compounds have a suitable A1/5_uff_E=277.56 −10.9
number of hydrogen bond donors (0–1 for nitrogen- A20_uff_E=282.50 −10.8
hydrogen and oxygen-hydrogen bonds) and hydrogen bond A33_uff_E=300.83 −10.5
acceptors (4–7 for nitrogen or oxygen atoms), which fall A49_uff_E=284.88 −10.5
within the recommended ranges (<5 and <10, respectively). A48_uff_E=343.12 −9.9
Their molecular weights range from 367.40 to 449.40 g/mol, A36_uff_E=283.54 −9.8
aligning with the guideline of 150 to 500 g/mol. The observed
topological polar surface area (TPSA) values range from A45_uff_E=414.27 −9.8
56.26 to 104 Å2, which also conform to the acceptable range Mefloquine −9.6
of 20 to 130 Å2. In addition, the number of rotatable bonds Piperaquine −9
in these compounds does not exceed 9. A34_uff_E=340.75 −8.7
According to a previous reported by Daina et al. , Artesunate −8.5
[23]
the negative log Kp values (−5.13 to −5.18) suggest that Doxycycline −8.5
compounds A20, A45, and A49 are predicted to have Tafenoquine −8.5
lower permeability through human skin compared to the Amodiaquine −8.4
other compounds. On the contrary, compounds A31, A36, Artemether −8.3
and A45 exhibit reduced gastrointestinal absorption rates.
These characteristics can be attributed to specific structural Lumefantrine −7.3
features, such as the presence of a trifluoromethyl group Primaquine −6.9
at position 6 in compound A31, a thiol group at position Chloroquine −6
8 in compound A36, and a combination of a methoxy
group at position 6 and a thiol group at position 3 in rates, except for A31, A36, and A45. Moreover, unlike
the methylphenoxyl ring. Notably, compound 5 displays the other lead compounds, compound 5 has the ability
permeability through the blood–brain barrier, unlike to penetrate the blood–brain barrier, as indicated in
the other lead compounds. Furthermore, compounds Table 5. All nine lead compounds demonstrate good
A31, A33, A34, and A36 are identified as substrates for oral bioavailability, with a value of 0.55, with only one
P-glycoprotein (P-gp) based on the studies conducted by permissible violation according to Lipinski et al.
[25]
Daina et al. Klopman et al. [23,24] . The inhibition of cytochrome P450 (CYP) isoenzymes
Out of the nine lead compounds, six, including is recognized as a major factor contributing to
compound 5, exhibited high gastrointestinal absorption pharmacokinetics-related drug–drug interactions . Such
[26]
Volume 7 Issue 1 (2024) 9 https://doi.org/10.36922/itps.0976