Page 52 - JCTR-10-1
P. 52
48 Gonda et al. | Journal of Clinical and Translational Research 2024; 10(1): 33-51
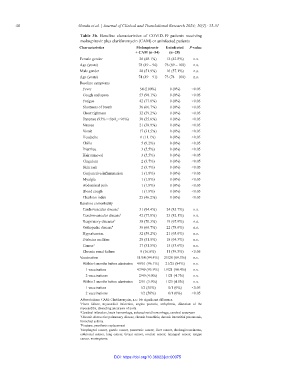
Table 3b. Baseline characteristics of COVID-19 patients receiving
molnupiravir plus clarithromycin (CAM) or uninfected patients
Characteristics Molnupiravir Uninfected P‑value
+ CAM (n=54) (n=28)
Female gender 26 (48.1%) 12 (42.8%) n.s.
Age (years) 78 (49 – 96) 76 (69 – 100) n.s.
Male gender 28 (51.9%) 16 (57.1%) n.s.
Age (years) 74 (49 – 91) 73 (74 – 100) n.s.
Baseline symptoms
Fever 54 (100%) 0 (0%) <0.05
Cough and sputa 53 (98.1%) 0 (0%) <0.05
Fatigue 42 (77.8%) 0 (0%) <0.05
Shortness of breath 36 (66.7%) 0 (0%) <0.05
Chest tightness 32 (59.2%) 0 (0%) <0.05
Dyspnea (93% < SpO < 96%) 30 (55.6%) 0 (0%) <0.05
2
Nausea 21 (38.9%) 0 (0%) <0.05
Vomit 17 (31.5%) 0 (0%) <0.05
Headache 6 (11.1%) 0 (0%) <0.05
Chills 5 (9.2%) 0 (0%) <0.05
Diarrhea 3 (5.5%) 0 (0%) <0.05
Hair removal 3 (5.5%) 0 (0%) <0.05
Cingulum 2 (3.7%) 0 (0%) <0.05
Skin rash 2 (3.7%) 0 (0%) <0.05
Conjunctiva inflammation 1 (1.8%) 0 (0%) <0.05
Myalgia 1 (1.8%) 0 (0%) <0.05
Abdominal pain 1 (1.8%) 0 (0%) <0.05
Blood cough 1 (1.8%) 0 (0%) <0.05
Charlson index 25 (46.2%) 0 (0%) <0.05
Baseline comorbidity
Cardiovascular disease 1 51 (94.4%) 24 (85.7%) n.s.
Cerebrovascular disease 2 42 (77.8%) 23 (82.1%) n.s.
Respiratory diseases 3 38 (70.3%) 19 (67.9%) n.s.
Orthopedic disease 4 36 (66.7%) 22 (78.6%) n.s.
Hypertension 32 (59.2%) 21 (65.6%) n.s.
Diabetes mellitus 28 (51.8%) 18 (64.3%) n.s.
Cancer 5 17 (31.5%) 15 (53.6%) n.s.
Chronic renal failure 9 (16.6%) 11 (39.3%) <0.05
Vaccination 51/54 (94.4%) 25/28 (89.3%) n.s.
Within 6 months before admission 49/51 (96.1%) 21/25 (84%) n.s.
1 vaccination 47/49 (95.9%) 19/21 (90.4%) n.s.
2 vaccinations 2/49 (4.0%) 1/21 (4.7%) n.s.
Within 3 months before admission 2/51 (3.9%) 1/25 (4.0%) n.s.
1 vaccination 1/2 (50%) 0/1 (0%) <0.05
2 vaccinations 1/2 (50%) 0/1 (0%) <0.05
Abbreviations: CAM: Clarithromycin, n.s.: No significant difference.
1 Heart failure, myocardial infarction, angina pectoris, arrhythmia, dilatation of the
myocarditis, dissecting aneurysm of aorta
2 Cerebral infarction, brain hemorrhage, subarachnoid hemorrhage, cerebral aneurysm
3 Chronic obstructive pulmonary disease, chronic bronchitis, chronic interstitial pneumonia,
bronchial asthma
4 Fracture, prosthesis replacement
5 Esophageal cancer, gastric cancer, pancreatic cancer, liver cancer, cholangiocarcinoma,
colorectal cancer, lung cancer, breast cancer, ovarian cancer, laryngeal cancer, tongue
cancer, meningioma.
DOI: https://doi.org/10.36922/jctr.00075