Page 54 - JCTR-10-1
P. 54
50 Gonda et al. | Journal of Clinical and Translational Research 2024; 10(1): 33-51
Supplementary Data 5
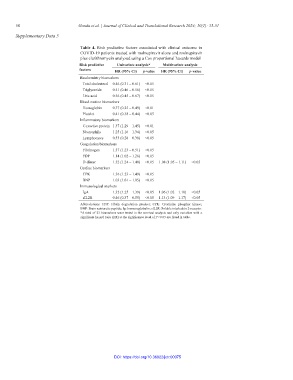
Table 4. Risk predictive factors associated with clinical outcome in
COVID-19 patients treated with molnupiravir alone and molnupiravir
plus clarithromycin analyzed using a Cox proportional hazards model
Risk predictive Univariate analysis* Multivariate analysis
factors HR (95% CI) p‑value HR (95% CI) p‑value
Biochemistry biomarkers
Total cholesterol 0.46 (0.31 – 0.61) <0.05
Triglyceride 0.51 (0.46 – 0.56) <0.05
Uric acid 0.56 (0.45 – 0.67) <0.05
Blood routine biomarkers
Hemoglobin 0.37 (0.25 – 0.49) <0.01
Platelet 0.41 (0.38 – 0.44) <0.05
Inflammatory biomarkers
C-reactive protein 1.37 (1.29 – 1.45) <0.01
Neutrophils 1.25 (1.16 – 1.34) <0.05
Lymphocytes 0.33 (0.28 – 0.38) <0.05
Coagulation biomarkers
Fibrinogen 1.37 (1.23 – 0.51) <0.05
FDP 1.14 (1.02 – 1.26) <0.05
D-dimer 1.32 (1.24 – 1.40) <0.05 1.08 (1.05 – 1.11) <0.05
Cardiac biomarkers
CPK 1.36 (1.23 – 1.49) <0.05
BNP 1.03 (1.01 – 1.05) <0.05
Immunological markers
IgA 1.32 (1.25 – 1.39) <0.05 1.06 (1.02 – 1.10) <0.05
sIL2R 0.46 (0.37 – 0.55) <0.05 1.13 (1.09 – 1.17) <0.05
Abbreviations: FDP: Fibrin degradation product; CPK: Creatinine phosphor kinase;
BNP: Brain natriuretic peptide; Ig: Immunoglobulin; sIL2R: Soluble interleukin 2-receptor.
*A total of 23 biomarkers were tested in the survival analysis and only variables with a
significant hazard ratio (HR) at the significance level of P<0.05 are listed in table.
DOI: https://doi.org/10.36922/jctr.00075