Page 119 - MSAM-2-3
P. 119
Materials Science in Additive Manufacturing SLA 3D printed triaxial nozzle
lines. A few clumps were observed while printing the grid 3.5. 3D Bioprinting and biocompatibility studies
outline, without impact in the shape fidelity. For IIFK, the The viability of cells on 3D bioprinting is an essential
resolution was mediocre, with less defined segmented lines. parameter of a successful process in tissue engineering and
The gelation was more inconsistent, with the presence of regenerative medicine . The availability of bioinks that
[32]
some clogs affecting thread continuity. support cellular growth and proliferation while allowing
Furthermore, to assess the nozzle’s capability to achieve proper cell function is crucial for a successful bioprinting
continuous hydrogel deposition in taller constructs, a process . In addition, the extrusion unit (nozzle) and its
[33]
hollow cylinder of 10 × 10 × 10 mm was printed using design are essential in having a high percentage of viable
3
IIZK (Figure 3C). A continuous thread was formed during cells in the printed constructs . This is partly due to
[34]
printing, suggesting the absence of clogs. The layers of the the shear stress experienced by the cells during printing,
construct were seamlessly deposited, with no apparent significantly reducing the viability rate [33,35] . To assess the
sagging and loss of contact. According to these results, the potential of our newly designed nozzle as an extrusion unit
nozzle shows promising potential to be used for hydrogel- for 3D bioprinting, we assessed cell viability at different
based 3D bioprinting, since it enables, for the most part, time points post-printing using live-dead staining. In
continuous thread extrusion and forms 3D structures of addition, to demonstrate the feasibility of our newly
varying dimensions, shapes, and complexity with good designed nozzle, two different peptide bioinks were used
resolution for two distinct peptide bioinks. in the 3D bioprinting process.
A
B C
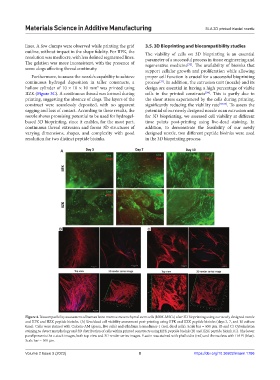
Figure 4. Biocompatibility assessment of human bone marrow mesenchymal stem cells (hBM-MSCs) after 3D bioprinting using our newly designed nozzle
and IIFK and IIZK peptide bioinks. (A) Live/dead cell viability assessment post-printing using IIFK and IIZK peptide bioinks (days 3, 7, and 10 culture
time). Cells were stained with Calcein-AM (green, live cells) and ethidium homodimer-1 (red, dead cells). Scale bar = 650 μm. (B and C) Cytoskeleton
staining to detect morphology and 3D distribution of cells within printed constructs using IIFK peptide bioink (B) and IIZK peptide bioink (C). The lower
panel presents the z-stack images, both top view and 3D render series images. F-actin was stained with phalloidin (red) and the nucleus with DAPI (blue).
Scale bar = 100 μm.
Volume 2 Issue 3 (2023) 8 https://doi.org/10.36922/msam.1786