Page 103 - OR-1-3
P. 103
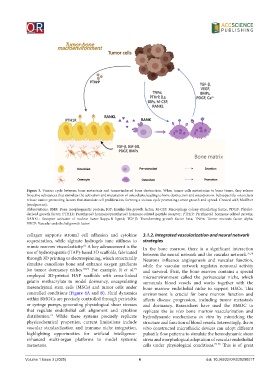
Figure 3. Vicious cycle between bone metastasis and tumor-induced bone destruction. When tumor cells metastasize to bone tissue, they release
bioactive substances that stimulate the activation and maturation of osteoclasts, leading to bone destruction and osteoporosis. Subsequently, osteoclasts
release tumor-promoting factors that stimulate cell proliferation, forming a vicious cycle promoting tumor growth and spread. Created with MedPeer
(medpeer.cn).
Abbreviations: BMP: Bone morphogenetic protein; IGF: Insulin-like growth factor; M-CSF: Macrophage colony-stimulating factor; PDGF: Platelet-
derived growth factor; PTH1R: Parathyroid hormone/parathyroid hormone-related peptide receptor; PTHrP: Parathyroid hormone-related protein;
RANKL: Receptor activator of nuclear factor kappa-B ligand; TGF-β: Transforming growth factor beta; TNFα: Tumor necrosis factor alpha;
VEGF: Vascular endothelial growth factor.
collagen supports stromal cell adhesion and cytokine 3.1.2. Integrated vascularization and neural network
sequestration, while alginate hydrogels tune stiffness to strategies
mimic marrow viscoelasticity. A key advancement is the In the bone marrow, there is a significant interaction
66
use of hydroxyapatite (HAP)-based 3D scaffolds, fabricated between the neural network and the vascular network. 75,76
through 3D printing or electrospinning, which structurally Neurons influence angiogenesis and vascular function,
simulate cancellous bone and enhance oxygen gradients while the vascular network regulates neuronal activity
71
for tumor dormancy niches. 72,73 For example, Ji et al. and survival. First, the bone marrow contains a special
employed 3D-printed HAP scaffolds with cross-linked microenvironment called the perivascular niche, which
gelatin methacrylate to model dormancy, encapsulating surrounds blood vessels and works together with the
mesenchymal stem cells (MSCs) and tumor cells under bone marrow endothelial niche to support HSCs. This
controlled conditions (Figure 4A and B). Fluid dynamics environment is crucial for bone marrow function and
within BMOCs are precisely controlled through peristaltic affects disease progression, including tumor metastasis
or syringe pumps, generating physiological shear stresses and dormancy. Researchers have used the BMOC to
that regulate endothelial cell alignment and cytokine replicate the in vivo bone marrow vascularization and
distribution. While these systems precisely replicate hydrodynamic mechanisms in vitro by mimicking the
74
physicochemical properties, current limitations include structure and function of blood vessels. Interestingly, the in
vascular standardization and immune niche integration, vitro constructed microfluidic devices can adopt different
highlighting opportunities for artificial intelligence- pulsatile flow patterns to simulate the hemodynamic shear
enhanced multi-organ platforms to model systemic stress and morphological adaptation of vascular endothelial
metastasis. cells under physiological conditions. 77-79 This is of great
Volume 1 Issue 3 (2025) 6 doi: 10.36922/OR025200017