Page 179 - AJWEP-22-5
P. 179
Alleviating aluminum toxicity
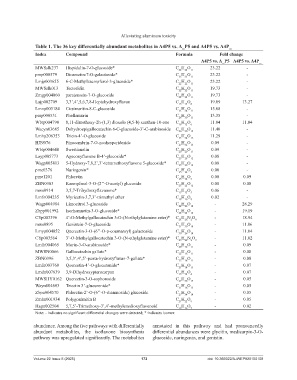
Table 1. The 36 key differentially abundant metabolites in A4P5 vs. A_P5 and A4P5 vs. A4P_
Index Compound Formula Fold change
A4P5 vs. A_P5 A4P5 vs. A4P_
MWSslk237 Hispidulin-7-O-glucoside* C H O 11 23.22 -
22
22
pmp000579 Diosmetin-7-O-galactoside* C H O 11 23.22 -
22
22
Lmjp003655 6-C-Methylkaempferol-3-glucoside* C H O 11 23.22 -
22
22
MWSslk013 Tectoridin C H O 11 19.73 -
22
22
Zmgp004060 paratensein-7-O-glucoside C H O 11 19.73 -
22
22
Lajp002709 3,3’,4’,5,6,7,8-Heptahydroxyflavan C H O 8 19.09 13.27
15
14
Lmnp003184 Cirsimaritin-8-C-glucoside C H O 11 15.68 -
24
23
pmp000531 Phellamurin C H O 13.35 -
26 30 11
Wbtp004798 8,11-dimethoxy-2h-(1,3) dioxolo (4,5-b) xanthen-10-one C H O 11.84 11.84
16 12 6
Wacyn03685 Dehydroepigallocatechin 6-C-glucoside-3’-C-arabinoside C H O 11.40 -
26 30 15
Lmhp206353 Tricin-4’-O-glucoside C H O 11.29 -
23 24 12
HJN076 Pinocembrin-7-O-neohesperidoside C H O 0.09 -
27 32 13
Wbtp004648 Swertianolin C H O 11 0.09 -
20
20
Layp005773 Ageconyflavone B-4’-glucoside* C H O 12 0.08 -
25
28
Wagp005813 5-Hydroxy-7,8,2’,3’-tetramethoxyflavone 5-glucoside* C H O 12 0.08 -
28
25
pme0376 Naringenin* C H O 5 0.08 -
12
15
pme1201 Phloretin C H O 5 0.08 0.09
15
14
ZBN0303 Kaempferol-3-O-(2’’-O-acetyl) glucoside C H O 12 0.08 0.08
23
22
mws0914 3,5,7-Trihydroxyflavanone* C H O 5 0.06 -
12
15
Lmfn004235 Myricetin-3,7,3’-trimethyl ether C H O 8 0.02 -
18
16
Wagp004984 Limocitrol 3-glucoside C H O 14 - 28.29
24
26
Zbpp001992 Isorhamnetin-3-O-glucoside* C H O 12 - 19.19
22
22
CYp003396 4’-O-Methylgallocatechin 3-O-(N-ethylglutamine ester)* C H N O - 18.94
23 28 2 9
mws0895 Genistein-7-O-glucoside C H O - 11.06
21 20 10
Lmyp004052 Quercetin-3-O-(6’’-O-p-coumaroyl) galactoside C H O - 11.04
30 26 14
CYp003564 3’-O-Methylgallocatechin 3-O-(N-ethylglutamine ester)* C H N O - 11.02
23 28 2 9
Lmfn004065 Morin-3-O-arabinoside* C H O - 0.09
20 18 11
MWSN0066 Gallocatechin gallate* C H O 11 - 0.08
18
22
ZBN0396 3,5,3’,4’,5’-penta-hydroxyflavan-7-gallate* C H O 11 - 0.08
18
22
Lmfn003760 Quercetin-4’-O-glucuronide* C H O 13 - 0.07
18
21
Lmdn007639 3,9-Dihydroxypterocarpan C H O 4 - 0.07
15
12
MWSHY0162 Quercetin-3-O-sophoroside C H O 17 - 0.05
27
30
Wayn004603 Tricetin 3’-glucuronide* C H O 13 - 0.05
18
21
Zbpn004570 Phloretin-2’-O-(6’’-O-rhamnoside) glucoside C H O 14 - 0.05
34
27
Zmhn001934 Polygonimitin B C H O 9 - 0.05
21
22
Hagn002504 5,7,5’-Trimethoxy-3’,4’-methylenedioxyflavonoid C H O 7 - 0.02
16
19
Note: - indicates no significant differential changes were detected; * indicates isomer.
abundance. Among the five pathways with differentially annotated in this pathway and had pronouncedly
abundant metabolites, the isoflavone biosynthesis differential abundances were glycitin, medicarpin-3-O-
pathway was upregulated significantly. The metabolites glucoside, naringenin, and genistin.
Volume 22 Issue 5 (2025) 173 doi: 10.366922/AJWEP025150108