Page 99 - AJWEP-22-6
P. 99
Adsorption desulfurization
A B C
D E F
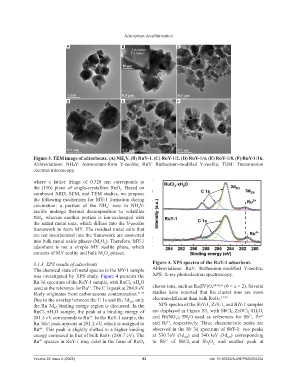
Figure 3. TEM image of adsorbents. (A) NH Y. (B) RuY-1. (C) RuY-1/2. (D) RuY-1/4. (E) RuY-1/8. (F) RuY-1/16.
4
Abbreviations: NH₄Y: Ammonium-form Y-zeolite; RuY: Ruthenium-modified Y-zeolite; TEM: Transmission
electron microscopy.
where a lattice fringe of 0.328 nm corresponds to
the (110) plane of single-crystalline RuO . Based on
2
combined XRD, SEM, and TEM studies, we propose
the following mechanism for MY-1 formation during
calcination: a portion of the NH ions in NH Y-
+
4
4
zeolite undergo thermal decomposition to volatilize
NH , whereas another portion is ion-exchanged with
3
the added metal ions, which diffuse into the Y-zeolite
framework to form MY. The residual metal salts that
are not incorporated into the framework are converted
into bulk metal oxide phases (M O ). Therefore, MY-1
x
y
adsorbent is not a simple MY zeolite phase, which
consists of MY zeolite and bulk M O phases.
x
y
3.1.3. XPS results of adsorbents Figure 4. XPS spectra of the RuY-1 adsorbent.
The chemical state of metal species in the MY-1 sample Abbreviations: RuY: Ruthenium-modified Y-zeolite;
was investigated by XPS study. Figure 4 presents the XPS: X-ray photoelectron spectroscopy.
Ru 3d spectrum of the RuY-1 sample, with RuCl ·xH O
2
3
used as the reference for Ru . The C 1s peak at 284.8 eV cluster ions, such as Ru(IV)Oₓ⁽⁴⁻²ˣ⁾⁺ (0 < x < 2). Several
3+
likely originates from carbonaceous contamination. 31,32 studies have reported that Ru cluster ions are more
Due to the overlap between the C 1s and Ru 3d , only electron-deficient than bulk RuO₂. 33-35
3/2
the Ru 3d binding energy region is discussed. In the XPS spectra of the SbY-1, ZrY-1, and BiY-1 samples
5/2
RuCl ·xH O sample, the peak at a binding energy of are displayed in Figure S3, with SbCl , ZrOCl ·8H O,
3
2
2
3
2
3+
4+
281.5 eV corresponds to Ru . In the RuY-1 sample, the and Bi(NO ) ·5H O used as references for Sb , Zr
3+
3 3
2
3+
Ru 3d₅/₂ peak appears at 281.2 eV, which is assigned to and Bi , respectively. Three characteristic peaks are
Ru ⁺. This peak is slightly shifted to a higher binding observed in the Sb 3d spectrum of SbY-1: two peaks
4
energy compared to that of bulk RuO₂ (280.7 eV). The at 530.7eV (3d ) and 540.1eV (3d ) corresponding
5/2
3/2
Ru ⁺ species in RuY-1 may exist in the form of RuOₓ to Sb of SbCl and Sb O and another peak at
3+
4
3
2
3,
Volume 22 Issue 6 (2025) 93 doi: 10.36922/AJWEP025250204