Page 63 - ARNM-2-3
P. 63
Advances in Radiotherapy
& Nuclear Medicine NCRT for T3N0M0 ESCC
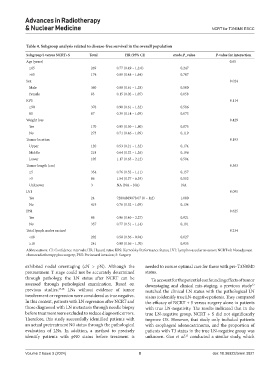
Table 4. Subgroup analysis related to disease‑free survival in the overall population
Subgroup S versus NCRT+S Total HR (95% CI) crude.P_value P‑value for interaction
Age (years) 0.65
≤65 269 0.77 (0.49 – 1.2.0) 0.247
>65 174 0.89 (0.48 – 1.64) 0.707
Sex 0.024
Male 360 0.88 (0.61 – 1.28) 0.500
Female 83 0.15 (0.02 – 1.07) 0.058
KPS 0.114
≥90 376 0.90 (0.61 – 1.32) 0.586
80 67 0.39 (0.14 – 1.09) 0.073
Weight loss 0.429
Yes 170 0.95 (0.50 – 1.80) 0.875
No 273 0.71 (0.46 – 1.09) 0.119
Tumor location 0.193
Upper 120 0.53 (0.21 – 1.32) 0.174
Middle 218 0.64 (0.32 – 1.26) 0.196
Lower 105 1.17 (0.65 – 2.12) 0.594
Tumor length (cm) 0.363
≤5 354 0.76 (0.52 – 1.11) 0.157
>5 86 1.54 (0.37 – 6.39) 0.552
Unknown 3 NA (NA – NA) NA
LVI 0.095
Yes 24 72004889078.07 (0 – Inf) 1.000
No 419 0.76 (0.52 – 1.09) 0.134
PNI 0.625
Yes 86 0.96 (0.40 – 2.27) 0.921
No 357 0.77 (0.51 – 1.14) 0.191
Total lymph nodes excised 0.234
<18 202 0.58 (0.36 – 0.94) 0.027
≥18 241 0.98 (0.56 – 1.70) 0.935
Abbreviations: CI: Confidence intervals; HR: Hazard ratio; KPS: Karnofsky Performance Status; LVI: Lymphovascular invasion; NCRT+S: Neoadjuvant
chemoradiotherapy plus surgery; PNI: Perineural invasion; S: Surgery.
exhibited nodal overstaging (cN > pN). Although the needed to ensure optimal care for those with pre-T3N0M0
pretreatment T stage could not be accurately determined status.
through pathology, the LN status after NCRT can be To account for the potential confounding effects of tumor
assessed through pathological examination. Based on downstaging and clinical mis-staging, a previous study
22
previous studies, 17,18 LNs without evidence of tumor matched the clinical LN status with the pathological LN
involvement or regression were considered as true negative. status to identify true LN-negative patients. They compared
In this context, patients with LN regression after NCRT and the efficacy of NCRT + S versus surgery alone in patients
those diagnosed with LN metastasis through needle biopsy with true LN-negativity. The results indicated that in the
before treatment were excluded to reduce diagnostic errors. true LN-negative group, NCRT + S did not significantly
Therefore, this study successfully identified patients with improve OS. However, that study only included patients
an actual pretreatment N0 status through the pathological with esophageal adenocarcinoma, and the proportion of
evaluation of LNs. In addition, a method to precisely patients with T3 status in the true LN-negative group was
23
identify patients with pN0 status before treatment is unknown. Gao et al. conducted a similar study, which
Volume 2 Issue 3 (2024) 8 doi: 10.36922/arnm.3821