Page 448 - IJB-10-1
P. 448
International Journal of Bioprinting Bioactive scaffold for necrosis bone repair
72 h suggested that more rBMSCs were crawling on the the HBPT had better in vitro osteogenesis results, and in
scaffold, indicating rapid cell growth and proliferation on the in vivo experiments, we used PT and HBPT as bone
the HBPT scaffold and good cell affinity of the scaffold. repair materials to evaluate the bone repair effect.
We then performed immunofluorescence staining and
Western blotting to observe the mechanism by which 3.3. Animal modeling and scaffold implantation
the HBPT scaffold contributes to osteogenesis. The Figure 4A depicts the flowchart of animal experiment.
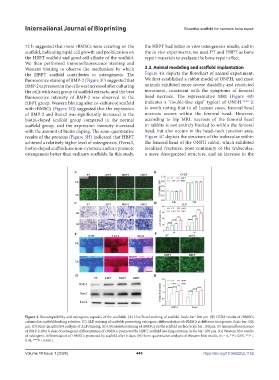
fluorescence staining of BMP-2 (Figure 3F) suggested that We first established a rabbit model of ONFH, and most
BMP-2 expression in the cells was increased after culturing animals exhibited more severe disability and restricted
the cells with each group of scaffold extracts, and the best movement, consistent with the symptoms of femoral
fluorescence intensity of BMP-2 was observed in the head necrosis. The representative MRI (Figure 4B)
HBPT group. Western blotting after co-culture of scaffold indicates a “double-line sign” typical of ONFH. 31,32 It
with rBMSCs (Figure 3G) suggested that the expression is worth noting that in all human cases, femoral head
of BMP-2 and Runx2 was significantly increased in the necrosis occurs within the femoral head. However,
biotin-doped scaffold group compared to the normal according to hip MRI, necrosis of the femoral head
scaffold group, and the expression intensity increased in rabbits is not entirely limited to within the femoral
with the amount of biotin doping. The semi-quantitative head, but also occurs in the head–neck junction area.
results of the proteins (Figure 3H) indicated that HBPT Figure 4C depicts the structure of the trabeculae within
achieved a relatively higher level of osteogenesis. Overall, the femoral head of the ONFH rabbit, which exhibited
biotin-doped scaffolds are non-cytotoxic and can promote localized fractures, poor continuity of the trabeculae,
osteogenesis better than ordinary scaffolds. In this study, a more disorganized structure, and an increase in the
Figure 3. Biocompatibility and osteogenic capacity of the scaffolds. (A) Live/Dead staining of scaffold. Scale bar: 200 μm. (B) CCK8 results of rBMSCs
cultured in scaffold leaching solution. (C) ALP staining of scaffolds promoting osteogenic differentiation of rBMSCs at different time points. Scale bar: 500
μm. (D) Semi-quantitative analysis of ALP staining. (E) Cytoskeletal staining of rBMSCs on the scaffold surface Scale bar: 100 μm. (F) Immunofluorescence
of BMP-2 after 6 days of osteogenic differentiation of rBMSCs promoted by HBPT scaffold leaching solution. Scale bar: 100 μm. (G) Western blot results
of osteogenic differentiation of rBMSCs promoted by scaffold after 6 days. (H) Semi-quantitative analysis of Western blot results. (n = 6, *P < 0.05, **P <
0.01, ***P < 0.001).
Volume 10 Issue 1 (2024) 440 https://doi.org/10.36922/ijb.1152