Page 35 - IJB-2-2
P. 35
Jia Min Lee, Swee Leong Sing, Edgar Yong Sheng Tan, et al.
bers with nanoscale features formed by electrospin- of biomaterials. It is critical to ensure that the lumen
ning [81] . Microscale geometries confine and direct cell of vasculature network does not collapse while the
growth towards anisotropic direction. However, these stiffness does not impede nutrient and waste transpor-
methods fall short in describing cell behavior in native tation across the network. Moreover, with comput-
environment. Therefore, there is a need to design 3D er-based technology, vascular system for complex or-
engineered tissue in order to utilize and integrate the gan manufacturing can be simulated and printed with-
previous findings into a 3D perspective. in a bioprinted construct [83] .
(3) Functioning Vasculature Network (4) Material Formulation
Sooppan et al. demonstrated the perfusion and Apart from designing materials to improve print fi-
anastomosis of a microchannel printed using polydi- delity in bioprinting, materials for engineering cardiac
methylsiloxane (PDMS) [82] . The integration of bio- tissue is needed to capture the physiological and functi-
printed vascular network with host tissue still remains onal properties of native cardiac tissues. Nanocompo-
hopeful. One of the major concerns is with the choice site hydrogel and electronics printing can be engineered
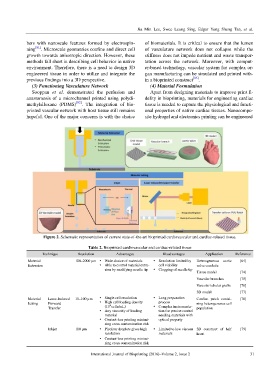
Figure 2. Schematic representation of current state-of-the-art bioprinted cardiovascular and cardiac-related tissue.
Table 2. Bioprinted cardiovascular and cardiac-related tissue
Technique Resolution Advantages Disadvantages Application Reference
Material 100–2000 µm Wide choice of materials Resolution limited by Heterogeneous aortic [64]
Extrusion Able to control material extru- cell viability valve conduits
sion by modifying needle tip Clogging of needle tip
Tissue model [74]
Vascular branches [75]
Vascular tubular grafts [76]
3D model [77]
Material Laser-Induced 10–100 µm Single cell resolution Long preparation Cardiac patch contai- [78]
Jetting Forward High cell loading density process ning heterogeneous cell
8
Transfer (10 cells/mL) Complex instrumenta- population
Any viscosity of loading tion for precise control
material needing materials with
Contact-less printing minimi- optical property
zing cross contamination risk
Inkjet 100 µm Picoliter droplets gives high Limited to low viscous 3D construct of half [79]
resolution materials heart
Contact-less printing minimi-
zing cross contamination risk
International Journal of Bioprinting (2016)–Volume 2, Issue 2 31