Page 248 - IJB-10-2
P. 248
International Journal of Bioprinting G40T60@WNT5A promotes osteoblast differentiation
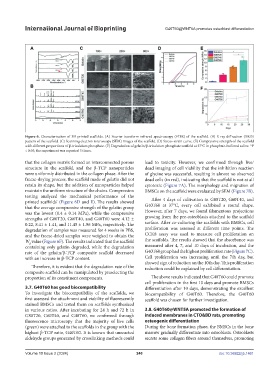
Figure 6. Characterization of 3D-printed scaffolds. (A) Fourier-transform infrared spectroscopy (FTIR) of the scaffold. (B) X-ray diffraction (XRD)
pattern of the scaffold. (C) Scanning electron microscopy (SEM) images of the scaffold. (D) Stress–strain curve. (E) Compressive strength of the scaffold
with different proportions of β-tricalcium phosphate. (F) Degradation of gelatin/β-tricalcium phosphate scaffold at 37°C in phosphate-buffered saline. *P
< 0.05; the experiment was repeated 3 times.
that the collagen matrix formed an interconnected porous lead to toxicity. However, we confirmed through live/
structure in the scaffold, and the β-TCP nanoparticles dead imaging of cell viability that the inhibition reaction
were uniformly distributed in the collagen phase. After the of glycine was successful, resulting in almost no observed
freeze-drying process, the scaffold made of gelatin did not dead cells (in red), indicating that the scaffold is not at all
retain its shape, but the addition of nanoparticles helped cytotoxic (Figure 7A). The morphology and migration of
maintain the uniform structure of the chains. Compression BMSCs on the scaffold were evaluated by SEM (Figure 7B).
testing analyzed the mechanical performance of the
printed scaffolds’ (Figure 6D and E). The results showed After 4 days of cultivation in G80T20, G60T40, and
that the average compressive strength of the gelatin group G40T60 at 37°C, every cell exhibited a round shape.
was the lowest (0.4 ± 0.14 MPa), while the compressive However, after 7 days, we found filamentous projections
strengths of G80T20, G60T40, and G40T60 were 4.12 ± growing from the pre-osteoblasts attached to the scaffold
0.22, 8.41 ± 1.41, and 11.45 ± 1.96 MPa, respectively. The surface. After co-culturing the scaffolds with BMSCs, cell
degradation of samples was measured for 4 weeks in PBS, proliferation was assessed at different time points. The
and the freeze-dried samples were weighed to obtain the CCK8 assay was used to measure cell proliferation on
W value (Figure 6F). The results indicated that the scaffold the scaffolds. The results showed that the absorbance was
d
containing only gelatin degraded, while the degradation measured after 4, 7, and 10 days of incubation, and the
rate of the gelatin/β-TCP composite scaffold decreased G40T60 group had the highest proliferation rate (Figure 7C).
with an increase in β-TCP content. Cell proliferation was increasing until the 7th day, but
showed sign of reduction on the 10th day. This proliferation
Therefore, it is evident that the degradation rate of the reduction could be explained by cell differentiation.
composite scaffold can be manipulated by preselecting the
proportion of its constituent components. The above results indicated that G40T60 could promote
cell proliferation in the first 10 days and promote BMSCs
3.7. G40T60 has good biocompatibility differentiation after 10 days, demonstrating the excellent
To investigate the biocompatibility of the scaffolds, we biocompatibility of G40T60. Therefore, the G40T60
first assessed the attachment and viability of fluorescently scaffold was chosen for further investigation.
stained BMSCs and tested them on scaffolds synthesized
in various ratios. After incubating for 24 h and 72 h in 3.8. G40T60@WNT5A promoted the formation of
G80T20, G60T40, and G40T60, we confirmed through induced membranes in CTO&BD rats, promoting
fluorescence microscopy that the majority of live cells osteogenic differentiation
(green) were attached to the scaffolds in the group with the During the bone formation phase, the BMSCs in the bone
highest β-TCP ratio, G40T60. It is known that unreacted marrow gradually differentiate into osteoblasts. Osteoblasts
aldehyde groups generated by crosslinking methods could secrete some collagen fibers around themselves, promoting
Volume 10 Issue 2 (2024) 240 doi: 10.36922/ijb.1461