Page 566 - IJB-10-2
P. 566
International Journal of Bioprinting OLS design for distal femur osseointegration
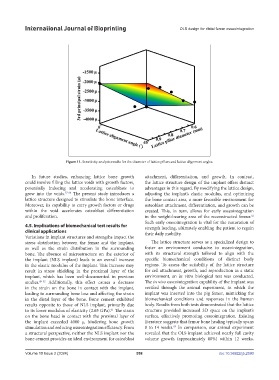
Figure 11. Sensitivity analysis results for the diameter of lattice pillars and lattice alignment angles.
In future studies, enhancing lattice bone growth attachment, differentiation, and growth. In contrast,
could involve filling the lattice voids with growth factors, the lattice structure design of the implant offers distinct
potentially inducing and accelerating osteoblasts to advantages in this regard. By modifying the lattice design,
grow into the voids. 57-60 The present study introduces a adjusting the implant’s elastic modulus, and optimizing
lattice structure designed to stimulate the bone interface. the bone contact area, a more favorable environment for
Moreover, its capability to carry growth factors or drugs osteoblast attachment, differentiation, and growth can be
within the void accelerates osteoblast differentiation created. This, in turn, allows for early osseointegration
and proliferation. in the weight-bearing area of the reconstructed femur.
62
Such early osseointegration is vital for the restoration of
4.5. Implications of biomechanical test results for strength loading, ultimately enabling the patient to regain
clinical applications their daily mobility.
Variations in implant structures and strengths impact the
stress distribution between the femur and the implant, The lattice structure serves as a specialized design to
as well as the strain distribution in the surrounding foster an environment conducive to osseointegration,
bone. The absence of microstructure on the exterior of with its structural strength tailored to align with the
the implant (NLS implant) leads to an overall increase specific biomechanical conditions of distinct body
in the elastic modulus of the implant. This increase may regions. To assess the suitability of the lattice structure
result in stress shielding in the proximal layer of the for cell attachment, growth, and reproduction in a static
implant, which has been well-documented in previous environment, an in vitro biological test was conducted.
studies. 49-52 Additionally, this effect causes a decrease The in vivo osseointegration capability of the implant was
in the strain on the bone in contact with the implant, verified through the animal experiment, in which the
leading to surrounding bone loss and affecting the strain implant was inserted into the pig femur, mimicking the
in the distal layer of the bone. Bone cement exhibited biomechanical conditions and responses in the human
results opposite to those of NLS implant, primarily due body. Results from both tests demonstrated that the lattice
to its lower modulus of elasticity (2.65 GPa). The strain structure provided increased 3D space on the implant’s
61
on the bone head in contact with the proximal layer of surface, effectively promoting osseointegration. Existing
the implant exceeded 4000 μ, hindering bone growth literature suggests that femur bone healing typically spans
stimulation and reducing osseointegration efficiency. From 8 to 14 weeks. In comparison, our animal experiment
63
a structural perspective, neither the NLS implant nor the revealed that the OLS implant achieved nearly full cavity
bone cement provides an ideal environment for osteoblast volume growth (approximately 80%) within 12 weeks.
Volume 10 Issue 2 (2024) 558 doi: 10.36922/ijb.2590