Page 35 - IJB-5-2
P. 35
Takagi D, et al.
A
B
C D
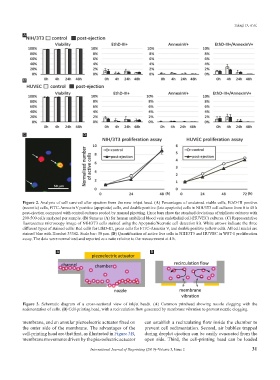
Figure 2. Analysis of cell survival after ejection from the new inkjet head. (A) Percentages of unstained viable cells, EthD-III positive
(necrotic) cells, FITC-Annexin V positive (apoptotic) cells, and double positive (late apoptotic) cells in NIH/3T3 cell cultures from 0 to 48 h
post-ejection compared with control cultures seeded by manual pipetting. Error bars show the standard deviations of triplicate cultures with
200-500 cells analyzed per sample. (B) Same as (A) for human umbilical blood vein endothelial cell (HUVEC) cultures. (C) Representative
fluorescence microscopy image of NIH/3T3 cells stained using the Apoptotic/Necrotic cell detection kit. White arrows indicate the three
different types of stained cells: Red cells for EthD-III, green cells for FITC-Annexin V, and double positive yellow cells. All cell nuclei are
stained blue with Hoechst 33342. Scale bar: 50 μm. (D) Quantification of active live cells in NIH/3T3 and HUVEC in WST-1 proliferation
assay. The data were normalized and reported as a ratio relative to the measurement at 4 h.
A B
Figure 3. Schematic diagram of a cross-sectional view of inkjet heads. (A) Common printhead showing nozzle clogging with the
sedimentation of cells. (B) Cell-printing head, with a recirculation flow generated by membrane vibration to prevent nozzle clogging.
membrane, and an annular piezoelectric actuator fixed on can establish a recirculating flow inside the chamber to
the outer side of the membrane. The advantages of the prevent cell sedimentation. Second, air bubbles trapped
cell-printing head are that first, as illustrated in Figure 3B, during droplet ejection can be easily evacuated from the
membrane movements driven by the piezoelectric actuator open side. Third, the cell-printing head can be loaded
International Journal of Bioprinting (2019)–Volume 5, Issue 2 31