Page 39 - IJB-5-2
P. 39
Takagi D, et al.
that despite a slight decrease at 24 h in NIH/3T3 cells droplets. This indicates that high accuracy control of cell
and between 24 and 48 h for HUVEC, the post-ejection number in each deposition is also achieved with the cell-
samples recovered normally and achieved a proliferation printing head.
rate similar to that of the manually seeded controls after
48-72 h. To further assess functional recovery in a more 3.7. Biofabrication Process for the Development
sensitive type of cells, a similar experiment was performed of 3D Mille-Feuille-like Constructs
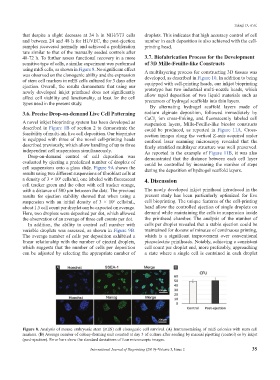
using mES cells, as shown in Figure 8. No significant effect A multilayering process for constructing 3D tissues was
was observed on the clonogenic ability and the expression developed, as described in Figure 10. In addition to being
of stem cell markers in mES cells cultured for 3 days after equipped with cell-printing heads, our inkjet bioprinting
ejection. Overall, the results demonstrate that using our prototype has two industrial multi-nozzle heads, which
newly developed inkjet printhead does not significantly
affect cell viability and functionality, at least for the cell allow rapid deposition of two liquid materials such as
types used in the present study. precursors of hydrogel scaffolds into thin layers.
By alternating hydrogel scaffold layers made of
3.6. Precise Drop-on-demand Live Cell Patterning sodium alginate deposition, followed immediately by
CaCl ion cross-linking, and fluorescently labeled cell
2
A novel inkjet bioprinting system has been developed as suspension layers, Mille-Feuille-like bicolor constructs
described in Figure 1B of section 2 to demonstrate the could be produced, as reported in Figure 11A. Cross-
feasibility of multi-ink live cell deposition. Our bioprinter section images along the vertical Z-axis acquired under
is equipped with three of the novel cell-printing heads confocal laser scanning microscopy revealed that the
described previously, which allow handling of up to three finely stratified multilayer structure was well preserved.
independent cell suspensions simultaneously. As reported in the example of Figure 11B, it was also
Drop-on-demand control of cell deposition was demonstrated that the distance between each cell layer
evaluated by ejecting a predefined number of droplets of could be controlled by increasing the number of steps
cell suspensions onto a glass slide. Figure 9A shows the during the deposition of hydrogel scaffold layers.
results using two different suspensions of fibroblast cells at
a density of 3 × 10 cells/ml, one labeled with fluorescent 4. Discussion
6
cell tracker green and the other with cell tracker orange,
with a distance of 500 μm between the dots. The previous The newly developed inkjet printhead introduced in the
results for ejection stability showed that when using a present study has been particularly optimized for live
suspension with an initial density of 3 × 10 cells/mL, cell bioprinting. The unique features of the cell-printing
6
about 1.5 cell count per droplet can be expected on average. head allow the controlled ejection of single droplets on
Here, two droplets were deposited per dot, which allowed demand while maintaining the cells in suspension inside
the observation of an average of three cell counts per dot. the printhead chamber. The analysis of the number of
In addition, the ability to control cell number with cells per droplet revealed that a stable ejection could be
variable droplets was assessed, as shown in Figure 9B. maintained for dozens of minutes of continuous printing,
The average number of cells per deposition exhibited a which is a significant improvement over conventional
linear relationship with the number of ejected droplets, piezoelectric printheads. Notably, achieving a consistent
which suggests that the number of cells per deposition cell count per droplet and, more preferably, approaching
can be adjusted by selecting the appropriate number of a state where a single cell is contained in each droplet
A B
Figure 8. Analysis of mouse embryonic stem (mES) cell clonogenic cell survival. (A) Immunostaining of mES colonies with stem cell
markers. (B) Average number of colony-forming unit counted at day 3 of culture after seeding by manual pipetting (control) or by inkjet
(post-ejection). Error bars show the standard deviations of four microscopic images.
International Journal of Bioprinting (2019)–Volume 5, Issue 2 35