Page 40 - IJB-5-2
P. 40
A novel inkjet system for live cell bioprinting
would be a major step toward the modeling of highly contrast to a previous report evaluating cell injury during
detailed 3D structures at the resolution of a single cell. laser bioprinting , no marked increase in necrotic nor in
[19]
The results showed that the new cell-printing head could apoptotic cells was observed from 0 to 48 h. The results
eject cells with a cell per droplet consistent with the are potentially because the level of stress induced by our
Poisson distribution profile, which indicated that the cell current process is lower than that in laser printing. The
suspension was maintained in a well-homogenized state cells recovered and proliferated normally after inkjet
by the mixing system. printing and were expected to maintain the integrity of
However, to achieve an even narrower distribution and their functions, including the clonogenicity of stem cells.
further increase the precision of deposition, it would be Our printheads bear several features are considerably
necessary to bring the state of random distribution closer different from common industrial inkjet heads and could
to a state of uniform distribution for the cells in suspension minimize cell damage. The open chamber structure and
inside the printhead chamber. This would require a strong the mixing system allow use over extended periods
repulsive force that acts between the cells, so that they without compromising gaseous exchange, whereas
are not brought close to each other, for example, by rapidly evacuating bubbles before their accumulation
introducing a polymer with a charge polarity that could increases the risk of damage following rupture . The
[20]
provide an electrostatic repulsive force between the cells. simplicity of the printhead chamber architecture and the
We are also investigating the potential of employing use of membrane vibration for droplet generation avert
additional optical cell count systems to further control the any excessive increase in liquid pressure and shear stress
number of cells per droplet. before ejection. Further investigations are required to
Regarding the suitability of using the new printheads assess the physical mechanisms that negatively influence
with living cells, analysis of cell viability and cell viability and function the most.
proliferation revealed that the ejected cells were not It is also worth noting, from a practical point of view,
significantly affected, even following the application particularly considering potential biomedical applications
of sensitive cells such as undifferentiated stem cells. In that the printhead chamber was intentionally kept simple
to ensure that low volumes of cell suspensions could be
A B loaded easily. Simplifying the procedures for loading
and exchanging cell suspensions could further reduce
the risks of environmental stress and contamination. This
could also be a major advantage when using rare cells
that are difficult to expand since our system does not
require filling ink cartridges or wasting cell suspensions
for maintenance.
Achieving a reliable ejection of living cells allowed
Figure 9. Drop-on-demand two-dimensional patterning evaluation us to subsequently experiment with more advanced
(A) Fluorescence microscope image of green- and red-labeled
NIH/3T3 cells deposited alternately at 500 μm intervals with two processes for the spatial positioning of cell-containing
cell-containing droplets ejected at each position. (B) Ability to droplets. We have demonstrated that an on-demand
control the number of cells based on the number of ejected droplets. patterning of cells over a flat surface is feasible with
Cell ink was formulated to contain one cell in two droplets. Error precise control of cell number at each deposition. Most
bars show the standard deviations. notably, the potential to draw intricate patterns with arrays
A B C
F E D
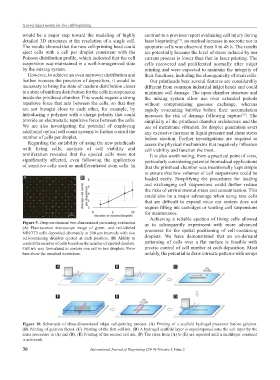
Figure 10. Schematic of three-dimensional inkjet cell-printing process. (A) Printing of a scaffold hydrogel precursor before gelation.
(B) Printing of gelation factor. (C) Printing of the first cell ink. (D) A hydrogel scaffold layer is superimposed onto the cell layer by the
same procedure in (A) and (B). (E) Printing of the second cell ink. (F) The steps from (A) to (E) are repeated until a multilayer construct
is achieved.
36 International Journal of Bioprinting (2019)–Volume 5, Issue 2