Page 201 - IJB-10-5
P. 201
International Journal of Bioprinting Structural design of D-surface scaffolds
could accelerate mineralization via released cytokines in
the osteogenic maturation process. 33
3.4. In vivo biocompatibility of scaffolds
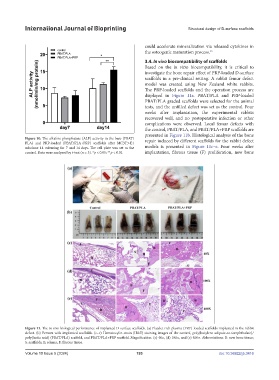
Based on the in vitro biocompatibility, it is critical to
investigate the bone repair effect of PRP-loaded D-surface
scaffolds in a pre-clinical setting. A rabbit femur defect
model was created using New Zealand white rabbits.
The PRP-loaded scaffolds and the operation process are
displayed in Figure 11a. PBAT/PLA and PRP-loaded
PBAT/PLA graded scaffolds were selected for the animal
tests, and the unfilled defect was set as the control. Four
weeks after implantation, the experimental rabbits
recovered well, and no postoperative infection or other
complications were observed. Local femur defects with
the control, PBAT/PLA, and PBAT/PLA+PRP scaffolds are
presented in Figure 11b. Histological analysis of the bone
Figure 10. The alkaline phosphatase (ALP) activity in the bare (PBAT/ repair induced by different scaffolds for the rabbit defect
PLA) and PRP-loaded (PBAT/PLA+PRP) scaffolds after MC3T3-E1
subclone 14 culturing for 7 and 14 days. The cell plate was set as the models is presented in Figure 11c–e. Four weeks after
control. Data were analyzed by t-test (n = 3). *p < 0.05; **p < 0.01. implantation, fibrous tissue (F) proliferation, new bone
Figure 11. The in vivo biological performance of implanted D-surface scaffolds. (a) Platelet-rich plasma (PRP)-loaded scaffolds implanted in the rabbit
defect. (b) Femurs with implanted scaffolds. (c–e) Hematoxylin-eosin (H&E) staining images of the control, poly(butylene adipate-co-terephthalate)/
poly(lactic acid) (PBAT/PLA) scaffold, and PBAT/PLA+PRP scaffold. Magnification: (c) 40×, (d) 100×, and (e) 400×. Abbreviations: B: new bone tissue;
S: scaffolds; E: edema; F: fibrous tissue.
Volume 10 Issue 5 (2024) 193 doi: 10.36922/ijb.3416