Page 63 - IJB-8-3
P. 63
Nguyen, et al.
investigation, the GF secreted from the HASMC layer- [ID], G100-3, Warner Instruments LLC, U.S.A.) for inlets
induced angiogenic sprouting. Gao et al. fabricated and one outer glass capillary (1160 µm internal diameter,
HUVEC single-layered single-vascular scaffold and G200-3, Warner Instruments LLC, U.S.A.) for an outlet.
demonstrated angiogenic sprouting using GF mixed Two glass tubes among the three 580 µm ID tubes were
collagen . These methods were not appropriate for tapered as approximately 200 µm using a puller (PC-10,
[19]
vascular network of the engineered thick tissue. Narishige, Japan) for the two inner core inlets. The other
In this study, we extruded two-vasculature- 580 µm ID tube supplied the outmost layer material to the
embedded scaffold and demonstrated angiogenesis for the 1160 µm ID tube without any tapering. All the four tubes
pre-vascularized tissue. The structure of scaffold consisted linked each other in a block of polydimethylsiloxane
of one hollow channel for flowing GF mixed media and (PDMS, Dow Corning Corporation, U.S.A.). The
one HUVEC core for a vascular channel (Figure 1). GF fabricated device was sterilized at 121°C for 15 min
gradient from the hollow channel induced angiogenic before biological experiments.
sprouting from the HUVEC vessel inside the generated
scaffold. This angiogenesis was compared at three 2.2. HUVEC culture
different culture conditions and analyzed quantitatively.
Effect of shear stress, perfusibility, cell viability, and core HUVEC was purchased from the American Type Culture
size were also evaluated. Collection (ATCC, U.S.A.) and cultured in vascular
cell basal medium (ATCC, U.S.A.) supplemented with
2. Materials and methods Endothelial Cell Growth Kit-VEGF (ATCC, U.S.A.).
Media were changed 3 times a week. They were cultured
2.1. Two-core laminar flow device in a humidified incubator at 37°C with 5% CO and were
2
A two-core laminar flow device was designed and passaged before reaching approximately 80% surface
fabricated based on our previous device (Figure 2) . It coverage. The cells within passage from 10 to 15 were
[20]
has three inner glass capillaries (580 µm inner diameter used in experiments.
A B
Figure 1. The schematic of the two-vasculature-embedded scaffold. (A) One core (the green one) consisted of 0.1 M calcium chloride
dihydrate (CaCl ) only for the hollow channel. Another core (the pink one) was made of HUVECs, 3 mg/mL type-1 collagen, and 0.1 M
2
CaCl for the vascular channel. The shell layer consisted of gelatin and sodium alginate. (B) The cross-sectional view of the two-vasculature-
2
embedded scaffold right after the formation and after the maturation.
A B C
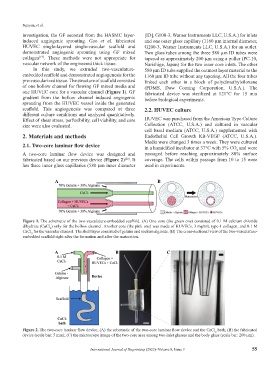
Figure 2. The two-core laminar flow device; (A) the schematic of the two-core laminar flow device and the CaCl bath; (B) the fabricated
2
device (scale bar: 5 mm); (C) the microscope image of the two-core area among two inlet glasses and the body glass (scale bar: 200 µm).
International Journal of Bioprinting (2022)–Volume 8, Issue 3 55