Page 198 - IJB-9-3
P. 198
International Journal of Bioprinting 3D-printed vascularized biofunctional scaffold
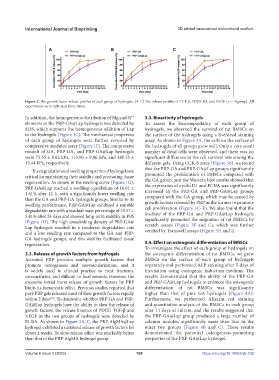
Figure 2. The growth factor release profiles of each group of hydrogels. (A–C) The release profiles of TGF-β, PDGF-BB, and VEGF (n = 3/group). All
experiments were replicated three times.
In addition, the homogeneous distribution of Mg and Si 3.3. Bioactivity of hydrogels
4+
elements in the PRP-GA@Lap hydrogels was detected by To assess the biocompatibility of each group of
EDS, which supports the homogeneous addition of Lap hydrogels, we observed the survival of rat BMSCs on
to the hydrogels (Figure 1C). The mechanical properties the surface of the hydrogels using a live/dead staining
of each group of hydrogels were further revealed by assay. As shown in Figure 3A, the cells on the surface of
compressive modulus assay (Figure 1F). The compressive the hydrogels of all groups grew well. Only a very small
moduli of GA, PRP-GA, and PRP-GA@Lap hydrogels number of dead cells were observed, and there was no
were 75.55 ± 6.62 kPa, 113.00 ± 9.86 kPa, and 180.55 ± significant difference in the cell survival rate among the
13.44 kPa, respectively. different gels. Using CCK-8 assay (Figure 3B), we found
The degradation and swelling properties of hydrogels are that the PRP-GA and PRP-GA@Lap groups significantly
critical for maintaining their stability and promoting tissue promoted the proliferation of BMSCs compared with
regeneration. As shown in the swelling curve (Figure 1G), the GA group, and the Western blot results showed that
PRP-GA@Lap reached a swelling equilibrium of 16.01 ± the expression of cyclin D1 and PCNA was significantly
1.61% after 12 h, with a significantly lower swelling rate increased in the PRP-GA and PRP-GA@Lap groups
than the GA and PRP-GA hydrogels groups. Similar to its compared with the GA group, which may be caused by
swelling performance, PRP-GA@Lap exhibited a suitable growth factors released by PRP in the former to promote
degradation rate with a residual mass percentage of 53.57 ± cell proliferation (Figure 3C–E). We also found that the
5.41% after 24 days and showed long-term stability in PBS leachate of the PRP-GA and PRP-GA@Lap hydrogels
(Figure 1H). The high crosslinking density of PRP-GA@ significantly promoted the migration of rat BMSCs by
Lap hydrogels resulted in a moderate degradation rate scratch assays (Figure 3F and G), which was further
and a low swelling rate compared to the GA and PRP- verified by Transwell assays (Figure 3H and I).
GA hydrogels groups, and this stability facilitated tissue
regeneration. 3.4. Effect on osteogenic differentiation of BMSCs
To investigate the effect of each group of hydrogels on
3.2. Release of growth factors from hydrogels the osteogenic differentiation of rat BMSCs, we grew
Activated PRP provides multiple growth factors that BMSCs on the surface of each group of hydrogels
promote osteogenesis and neovascularization, and it separately and performed ALP staining after 5 days of
is widely used in clinical practice to treat fractures, incubation using osteogenic induction medium. The
osteoarthritis, and difficult-to-heal wounds. However, the results demonstrated that the ability of the PRP-GA
excessive initial burst release of growth factors by PRP and PRP-GA@Lap hydrogels to promote the osteogenic
limits its therapeutic effect. Previous studies reported that differentiation of rat BMSCs was significantly
pure PRP gels released most of their growth factors rapidly higher than that of pure GA hydrogels (Figure 4A).
within 2 days . To determine whether PRP-GA and PRP- Furthermore, we performed Alizarin red staining
[30]
GA@Lap hydrogels have the ability to slow the release of and quantitative analysis of the BMSCs in each group
growth factors, the release kinetics of PDGF, TGF-β and after 14 days of culture, and the results suggested that
VEGF in the two groups of hydrogels were detected by the PRP-GA@Lap group produced a large number of
ELISA. As shown in Figure 2A–C, the PRP-AlgMA@Lap calcium nodules, significantly more than that in the
hydrogel exhibited a sustained release of growth factors for other two groups (Figure 4B and C). These results
about 2 weeks. Its slow-release effect was markedly better demonstrated the potential osteogenesis-promoting
than that of the PRP-AlgMA hydrogel group. properties of the PRP-GA@Lap hydrogel.
Volume 9 Issue 3 (2023) 190 https://doi.org/10.18063/ijb.702