Page 201 - IJB-9-3
P. 201
International Journal of Bioprinting 3D-printed vascularized biofunctional scaffold
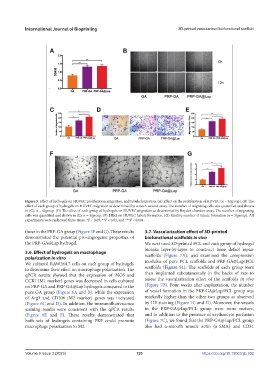
Figure 5. Effect of hydrogels on HUVEC proliferation, migration, and tubule formation. (A) Effect on the proliferation of HUVEC (n = 3/group). (B) The
effect of each group of hydrogels on HUVEC migration as determined by scratch-wound assay. The number of migrating cells was quantified and shown
in (C); n = 4/group. (D) The effect of each group of hydrogels on HUVEC migration as determined by Boyden chamber assay. The number of migrating
cells was quantified and shown in (E); n = 3/group. (F) Effect on HUVEC tubule formation. (G) Relative number of tubule formation (n = 3/group). All
experiments were replicated three times. *P < 0.05, **P < 0.01, and ***P < 0.001.
those in the PRP-GA group (Figure 5F and G). These results 3.7. Vascularization effect of 3D-printed
demonstrated the potential pro-angiogenic properties of biofunctional scaffolds in vivo
the PRP-GA@Lap hydrogel. We next used 3D-printed PCL and each group of hydrogel
bioinks layer-by-layer to construct bone defect repair
3.6. Effect of hydrogels on macrophage scaffolds (Figure 7A), and examined the compression
polarization in vitro
We cultured RAW264.7 cells on each group of hydrogels modulus of pure PCL scaffolds and PRP-GA@Lap/PCL
to determine their effect on macrophage polarization. The scaffolds (Figure S1). The scaffolds of each group were
qPCR results showed that the expression of iNOS and then implanted subcutaneously in the backs of rats to
CCR7 (M1 marker) genes was decreased in cells cultured assess the vascularization effect of the scaffolds in vivo
on PRP-GA and PRP-GA@Lap hydrogels compared to the (Figure 7B). Four weeks after implantation, the number
pure GA group (Figure 6A and B), while the expression of vessel formation in the PRP-GA@Lap/PCL group was
of Arg1 and CD206 (M2 marker) genes was increased markedly higher than the other two groups as observed
(Figure 6C and D). In addition, the immunofluorescence by HE staining (Figure 7C and D). Moreover, the vessels
staining results were consistent with the qPCR results in the PRP-GA@Lap/PCL group were more mature,
(Figure 6E and F). These results demonstrated that and in addition to the presence of erythrocyte perfusion
both sets of hydrogels containing PRP could promote (Figure 7C), we found that the PRP-GA@Lap/PCL group
macrophage polarization to M2. also had α-smooth muscle actin (α-SMA) and CD31
Volume 9 Issue 3 (2023) 193 https://doi.org/10.18063/ijb.702