Page 114 - IJB-9-6
P. 114
International Journal of Bioprinting Affordable temperature-controlled bioprinter
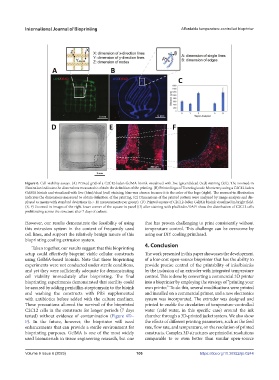
Figure 6. Cell viability assays. (A) Printed grid of a C2C12-laden GelMA bioink visualized with live (green)/dead (red) staining (left). The zoomed-in
illustration indicates the dimensions measured to obtain the definition of the printing. (B) Printed logo of Tecnologico de Monterrey using a C2C12-laden
GelMA bioink and visualized with live (blue)/dead (red) staining; blue was chosen because it is the color of the logo (right). The zoomed-in illustration
indicates the dimensions measured to obtain definition of the printing. (C) Dimensions of the printed pattern were analyzed by image analysis and dis-
played as means with standard deviations (n > 11 measurements per group). (D) Printed square of C2C12-laden GelMA bioink visualized in bright field.
(E, F) Zoomed-in images of the right lower corner of the square in panel (D) after staining with phalloidin/DAPI show the distribution of C2C12 cells
proliferating across the structure after 7 days of culture.
However, our results demonstrate the feasibility of using that has proven challenging to print consistently without
this extrusion system in the context of frequently used temperature control. This challenge can be overcome by
cell lines, and support the relatively benign nature of this using our DiY cooling printhead.
bioprinting cooling extrusion system.
4. Conclusion
Taken together, our results suggest that this bioprinting
setup could effectively bioprint viable cellular constructs The work presented in this paper showcases the development
using GelMA-based bioinks. Note that these bioprinting of a low-cost open-source bioprinter that has the ability to
experiments were not conducted under sterile conditions, provide precise control of the printability of inks/bioinks
and yet they were sufficiently adequate for demonstrating by the inclusion of an extruder with integrated temperature
cell viability immediately after bioprinting. The final control. This is done by converting a commercial 3D printer
bioprinting experiments demonstrated that sterility could into a bioprinter by employing the strategy of “printing your
be assured by adding penicillin-streptomycin to the bioink own printer.” To do this, several modifications were printed
and washing the constructs with PBS supplemented and installed on a commercial printer, and a new electronics
with antibiotics before added with the culture medium. system was incorporated. The extruder was designed and
These precautions allowed the survival of the bioprinted printed to enable the circulation of temperature-controlled
C2C12 cells in the constructs for longer periods (7 days water (cold water, in this specific case) around the ink
tested) without evidence of contamination (Figure 6D– chamber through a 3D-printed jacket system. We also show
F). In the future, however, the bioprinter will need the effects of different printing parameters, such as the feed
enhancements that can provide a sterile environment for rate, flow rate, and temperature, on the resolution of printed
bioprinting purposes. GelMA is one of the most widely constructs. Complex 3D structures are printed at resolutions
used biomaterials in tissue engineering research, but one comparable to or even better than similar open-source
Volume 9 Issue 6 (2023) 106 https://doi.org/10.36922/ijb.0244