Page 170 - IJB-9-6
P. 170
International Journal of Bioprinting 3D printing in gastroenterology
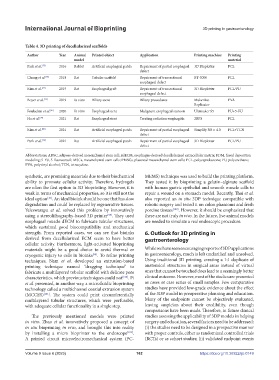
Table 4. 3D printing of decellularized scaffolds
Author Year Animal Printed object Application Printing machine Printing Printing technique Seeded cells Extracellular Bioreactor Results
model material matrix
Park et al. [75] 2016 Rabbit Artificial esophageal patch Repairment of partial esophageal 3D Bioplotter PCL Extrusion Rabbit MSCs Fibrin, thrombin None Better cell regeneration in MSC group
defect
Chung et al. [76] 2018 Rat Tubular scaffold Repairment of transectional BT-3000 PCL 3D printing & None None Omentum Better cell regeneration in MSC group
esophageal defect electrospinning
Kim et al. [77] 2019 Rat Esophageal graft Repairment of transectional 3D Bioplotter PCL/PU 3D printing & Human MSCs None Custom-made & omentum Satisfactory tissue regeneration with both
esophageal defect electrospinning bioreactors
Boyer et al. [95] 2019 In vitro Biliary stent Biliary procedures MakerBot PVA N/A Human PMSCs, human Collagen Growth medium Satisfactory cholangiocytes coating
Replicator primary cholangiocytes
Fouladian et al. [81] 2020 In vitro Esophageal stent Malignant esophageal stenosis Ultimaker S5 PU+5-FU FDM None None None Sustained release of 5-FU over 110 days
Ha et al. [79] 2021 Rat Esophageal stent Treating radiation esophagitis 2RPS PCL Extrusion None EdECM-based None Rapid resolution of inflammatory response
hydrogel
Kim et al. [80] 2021 Rat Artificial esophageal patch Repairment of partial esophageal Simplify 3D v. 4.0 PCL+TCN Extrusion None None None Better tissue regeneration and antibacterial
defect activity
Park et al. [78] 2021 Rat Artificial esophageal patch Repairment of partial esophageal 3D Bioplotter PCL/PU 3D printing & ADSC Matrigel & Growth medium Better cell regeneration in ADSC group
defect electrospinning fibronectin
Abbreviations: ADSC, adipose-derived mesenchymal stem cell; EdECM, esophagus-derived decellularized extracellular matrix; FDM, fused deposition
modeling; 5-FU, 5-fluorouracil; MSCs, mesenchymal stem cells; PMSCs, placental mesenchymal stem cells; PCL, polycaprolactone; PU, polyurethane;
PVA, polyvinyl alcohol; TCN, tetracycline.
synthetic, are promising materials due to their biochemical MEMS) technique was used to build the printing platform.
ability to promote cellular activity. Therefore, hydrogels They tested it by bioprinting a gelatin–alginate scaffold
are often the first option in 3D bioprinting. However, it is with human gastric epithelial and smooth muscle cells to
weak in terms of mechanical properties, so it is still not the repair a wound on a stomach model. Recently, Thai et al.
ideal option . An ideal bioink should be one that has slow also reported an in situ 3DP technique compatible with
[10]
degradation and could be replaced by regenerative tissues. robotic surgery and tested it on colon phantoms and fresh
Yeleswarapu et al. solved this problem by innovatively porcine tissues [103] . However, it should be emphasized that
using a stereolithography-based 3D printer . They used these are not truly in vivo. In the future, live animal models
[98]
esophageal muscle dECM to fabricate tubular structures, are needed to simulate a real endoscopic procedure.
which sustained good biocompatibility and mechanical
strength. From reported cases, we can see that bioinks 6. Outlook for 3D printing in
derived from decellularized ECM seem to have better gastroenterology
cellular activity. Furthermore, light-activated bioprinting
materials might be a good choice to avoid thermal or While we have seen encouraging reports of 3DP applications
cryogenic injury to cells in bioinks . To refine printing in gastroenterology, much is left unclarified and unsolved.
[99]
techniques, Nam et al. developed an extrusion-based Using traditional 3D printing, creating a 1:1 duplicate of
printing technique named “dragging technique” to anatomical structures in surgical areas instead of virtual
fabricate a multilayered tubular scaffold with delicate pore ones that cannot be touched does lead to a seemingly better
characteristics, which previous techniques could not [100] . Pi clinical outcome. However, most of the studies are presented
et al. presented, in another way, a microfluidic bioprinting as cases or case series of small samples. Few comparative
technology called a multichannel coaxial extrusion system studies have provided low-grade evidence about the effect
(MCCES) [101] . The system could print circumferentially of the 3DP model in preoperative planning and education.
multilayered tubular structures, which were perfusable, Many of the endpoints cannot be objectively evaluated,
with adequate cellular functionality in a single step. leaving suspicion about their credibility, even though
comparisons have been made. Therefore, in future clinical
The previously mentioned models were printed studies assessing the applicability of 3DP models in helping
in vitro. Zhao et al. innovatively proposed a concept of surgery and education, several factors need to be addressed:
in situ bioprinting in vivo, and brought this into reality (i) the studies need to be designed in a prospective manner
by installing a micro bioprinter to the endoscope [102] . with proper controls, either as randomized controlled trials
A printed circuit microelectromechanical system (PC- (RCTs) or as cohort studies; (ii) validated endpoint events
Volume 9 Issue 6 (2023) 162 https://doi.org/10.36922/ijb.0149