Page 410 - IJB-9-6
P. 410
International Journal of Bioprinting Biofabrication for islet transplantation
thereby opening new possibilities for prolonged stem 4. Biofabrication strategies for islet trans-
cell culture and differentiation . Sackett et al. presented plantation
[72]
a pioneering approach for the efficient decellularization
and elimination of lipids from the human pancreata . Despite the use of biological materials, there are numerous
[73]
They extensively evaluated the structure and composition obstacles to islet delivery in terms of manufacturing
of the delipidized pancreatic dECM (Figure 4C) and processes. During the encapsulation of islets into
demonstrated the elimination of human leukocyte antigen biomaterials and transplantation to the exact location, the
(HLA) from decellularized materials, thereby obviating islets are exposed to external forces. For example, severe
[73]
potential immune reactions (Figure 4D) . Berkova et physical forces can have fatal effects on cells encapsulated
al. established a viable model to evaluate the potential of in biomaterials, resulting in cell death owing to potential
decellularized pancreatic skeleton (Figure 4E) as a matrix damage to cell membranes. Using optimal biofabrication
for islet graft transplantation into the omentum (Figure 4F) methods, multiple cells and biomaterials can be readily
[74] . The transplanted islets maintained their morphology integrated into a concrete islet delivery construct. To
and position within the omentum and remained integrated overcome these challenges, various biofabrication methods
within the skeleton (Figure 4G). They also verified islet have been adopted, including conventional scaffold
viability and sustained insulin secretion in syngeneic fabrication methods, electrospinning, microfabrication,
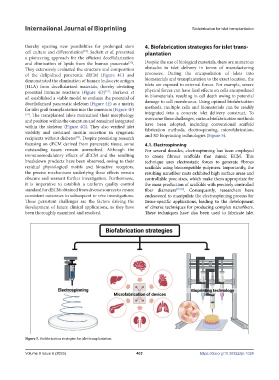
and 3D bioprinting technologies (Figure 5).
recipients without diabetes . Despite promising research
[74]
focusing on dECM derived from pancreatic tissue, some 4.1. Electrospinning
outstanding issues remain unresolved. Although the For several decades, electrospinning has been employed
immunomodulatory effects of dECM and the resulting to create fibrous scaffolds that mimic ECM. This
breakdown products have been observed, owing to their technique uses electrostatic forces to generate fibrous
residual physiological motifs and bioactive receptors, scaffolds using biocompatible polymers. Importantly, the
the precise mechanisms underlying these effects remain resulting nanofiber mats exhibited high surface areas and
obscure and warrant further investigation. Furthermore, controllable pore sizes, which make them appropriate for
it is imperative to establish a uniform quality control the mass production of scaffolds with precisely controlled
standard for dECM obtained from diverse sources to ensure fiber diameters [75,76] . Consequently, researchers have
consistent outcomes in subsequent in vivo investigations. endeavored to manipulate the electrospinning process for
These persistent challenges are the factors driving the tissue-specific applications, leading to the development
development of future clinical applications, as they have of diverse techniques for producing complex nanofibers.
been thoroughly examined and resolved. These techniques have also been used to fabricate islet
Figure 5. Biofabrication strategies for islet transplantation.
Volume 9 Issue 6 (2023) 402 https://doi.org/10.36922/ijb.1024