Page 408 - v11i4
P. 408
International Journal of Bioprinting Bioprinted liver dECM/GelMA tumor model
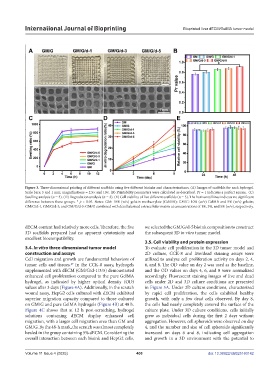
Figure 3. Three-dimensional printing of different scaffolds using five different bioinks and characterizations. (A) Images of scaffolds for each hydrogel.
Scale bars: 5 and 1 mm; magnifications = 2.5× and 10×. (B) Printability parameters were calculated as described. Pr = 1 indicates a perfect square. (C)
Swelling analysis (n = 3). (D) Degradation analysis (n = 3). (E) Cell viability of five different scaffolds (n = 3). The horizontal lines indicate no significant
difference between those groups. * p < 0.05. Notes: GM: 10% (w/v) gelatin methacrylate (GelMA); GM/G: 10% (w/v) GelMA and 5% (w/v) gelatin;
GM/G/d-1, GM/G/d-3, and GM/G/d-5: GM/G combined with decellularized extracellular matrix at concentrations of 1%, 3%, and 5% (w/v), respectively.
dECM content had relatively more cells. Therefore, the five we selected the GM/G/d-5 bioink composition to construct
3D scaffolds prepared had no apparent cytotoxicity and the subsequent 3D in vitro tumor model.
excellent biocompatibility.
3.5. Cell viability and protein expression
3.4. In vitro three-dimensional tumor model To evaluate cell proliferation in the 3D tumor model and
construction and assays 2D culture, CCK-8 and live/dead staining assays were
Cell migration and growth are fundamental behaviors of utilized to analyze cell proliferation activity on days 2, 4,
tumor cells and tissues. In the CCK-8 assay, hydrogels 6, and 8. The OD value on day 2 was used as the baseline,
49
supplemented with dECM (GM/G/d-1/3/5) demonstrated and the OD values on days 4, 6, and 8 were normalized
enhanced cell proliferation compared to the pure GelMA accordingly. Fluorescent staining images of live and dead
hydrogel, as indicated by higher optical density (OD) cells under 2D and 3D culture conditions are presented
values after 3 days (Figure 4A). Additionally, in the scratch in Figure 5A. Under 2D culture conditions, characterized
wound assay, HepG2 cells cultured with dECM exhibited by rapid cell proliferation, the cells exhibited healthy
superior migration capacity compared to those cultured growth, with only a few dead cells observed. By day 8,
on GM/G and pure GelMA hydrogels (Figure 4B) at 48 h. the cells had nearly completely covered the surface of the
Figure 4C shows that at 12 h post-scratching, hydrogel culture plate. Under 3D culture conditions, cells initially
solutions containing dECM display enhanced cell grew as individual cells during the first 2 days without
migration, with a larger cell migration area than GM and aggregation. However, cell spheroids were observed on day
GM/G. By the 48-h mark, the scratch was almost completely 4, and the number and size of cell spheroids significantly
healed in the group containing 5% dECM. Considering the increased on days 6 and 8, indicating cell aggregation
overall interaction between each bioink and HepG2 cells, and growth in a 3D environment with the potential to
Volume 11 Issue 4 (2025) 400 doi: 10.36922/IJB025160142