Page 72 - IMO-2-1
P. 72
Innovative Medicines & Omics Flavonoids against glycosidic hydrolase
A B
C D
E
F
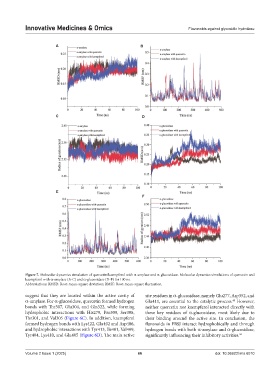
Figure 7. Molecular dynamics simulation of quercetin/kaempferol with α-amylase and α-glucosidase. Molecular dynamics simulations of quercetin and
kaempferol with α-amylase (A-C) and α-glucosidase (D-F) for 100 ns.
Abbreviations: RMSD: Root-mean-square deviation; RMSF: Root-mean-square fluctuation.
suggest that they are located within the active cavity of site residues in α-glucosidase, namely Glu277, Asp352, and
α-amylase. For α-glucosidase, quercetin formed hydrogen Glu411, are essential to the catalytic process. However,
49
bonds with Thr307, Glu304, and Gln322, while forming neither quercetin nor kaempferol interacted directly with
hydrophobic interactions with His279, Pro309, Ser308, these key residues of α-glucosidase, most likely due to
Thr301, and Val305 (Figure 6C). In addition, kaempferol their binding around the active site. In conclusion, the
formed hydrogen bonds with Lys422, Glu402 and Asp406, flavonoids in FBSJ interact hydrophobically and through
and hydrophobic interactions with Tyr413, Ile401, Val409, hydrogen bonds with both α-amylase and α-glucosidase,
Tyr404, Lys410, and Glu405 (Figure 6D). The main active significantly influencing their inhibitory activities. 50
Volume 2 Issue 1 (2025) 66 doi: 10.36922/imo.6010