Page 71 - IMO-2-1
P. 71
Innovative Medicines & Omics Flavonoids against glycosidic hydrolase
A
B
C
D
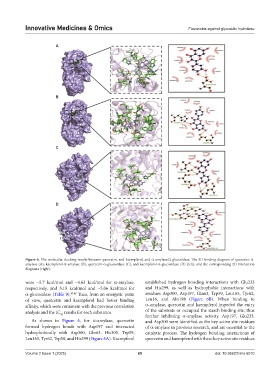
Figure 6. The molecular docking results between quercetin, and kaempferol, and α-amylase/α-glucosidase. The 3D binding diagram of quercetin-α-
amylase (A), kaempferol-α-amylase (B), quercetin-α-glucosidase (C), and kaempferol-α-glucosidase (D) (left), and the corresponding 2D interaction
diagrams (right).
were −5.7 kcal/mol and −4.63 kcal/mol for α-amylase, established hydrogen bonding interactions with Glu233
respectively, and 5.15 kcal/mol and −5.06 kcal/mol for and His299, as well as hydrophobic interactions with
α-glucosidase (Table 9). 47,48 Thus, from an energetic point residues Asp300, Asp197, Gln63, Trp59, Leu165, Tyr62,
of view, quercetin and kaempferol had better binding Leu16, and Aln198 (Figure 6B). When binding to
affinity, which were consistent with the previous correlation α-amylase, quercetin and kaempferol impeded the entry
analysis and the IC results for each substance. of the substrate or occupied the starch binding site, thus
50
further inhibiting α-amylase activity. Asp197, Glu233,
As shown in Figure 6, for α-amylase, quercetin and Asp300 were identified as the key active site residues
formed hydrogen bonds with Asp197 and interacted of α-amylase in previous research, and are essential to the
hydrophobically with Asp300, Gln63, His305, Trp59, catalytic process. The hydrogen bonding interactions of
Leu165, Tyr62, Trp58, and His299 (Figure 6A). Kaempferol quercetin and kaempferol with these key active site residues
Volume 2 Issue 1 (2025) 65 doi: 10.36922/imo.6010