Page 24 - IJB-10-4
P. 24
International Journal of Bioprinting PAI for 3D bioprinted constructs
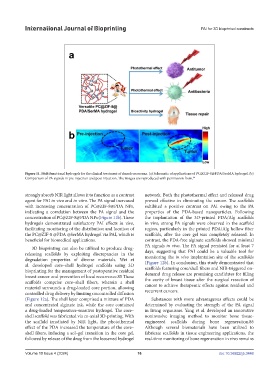
Figure 11. Multifunctional hydrogels for the clinical treatment of chondrosarcoma. (a) Schematic of applications of PC@ZIF-8@PDA/SerMA hydrogel. (b)
Comparison of PA signals in pre-injection and post-injection. The images are reproduced with permission from. 84
strongly absorb NIR light allows it to function as a contrast network. Both the photothermal effect and released drug
agent for PAI in vivo and in vitro. The PA signal increased proved effective in eliminating the cancer. The scaffolds
with increasing concentration of PC@ZIF-8@PDA NPs, exhibited a positive contrast on PAI owing to the PA
indicating a correlation between the PA signal and the properties of the PDA-based nanoparticles. Following
concentration of PC@ZIF-8@PDA NPs (Figure 11b). These the implantation of the 3D-printed PDA/Alg scaffolds
hydrogels demonstrated satisfactory PAI effects in vivo, in vivo, strong PA signals were observed in the scaffold
facilitating monitoring of the distribution and location of region, particularly in the printed PDA/Alg hollow fiber
the PC@ZIF-8 @PDA @SerMA hydrogel via PAI, which is scaffolds, after the core gel was completely released. In
beneficial for biomedical applications. contrast, the PDA-free alginate scaffolds showed minimal
PA signals in vivo. The PA signal persisted for at least 7
3D bioprinting can also be utilized to produce drug-
releasing scaffolds by exploiting discrepancies in the days, suggesting that PAI could be a valuable tool for
monitoring the in vivo implantation site of the scaffolds
degradation properties of diverse materials. Wei et (Figure 12b). In conclusion, this study demonstrated that
al. developed core–shell hydrogel scaffolds using 3D scaffolds featuring core/shell fibers and NIR-triggered on-
bioprinting for the management of postoperative residual demand drug release are promising candidates for filling
breast cancer and prevention of local recurrence.85 These the cavity of breast tissue after the surgical resection of
scaffolds comprise core–shell fibers, wherein a shell cancer to achieve therapeutic effects against residual and
material surrounds a drug-loaded core portion, allowing recurrent cancers.
controlled drug delivery by limiting uncontrolled diffusion
(Figure 12a). The shell layer comprised a mixture of PDA Substances with more advantageous effects could be
and concentrated alginate ink, while the core contained determined by evaluating the strength of the PA signal
a drug-loaded temperature-sensitive hydrogel. The core– in living organisms. Yang et al. developed an innovative
shell scaffold was fabricated via co-axial 3D-printing. With noninvasive imaging method to monitor bone tissue-
the scaffold irradiated by NIR light, the photothermal engineered scaffolds during bone regeneration.86
effect of the PDA increased the temperature of the core– Although several biomaterials have been utilized to
shell fibers, inducing a sol–gel transition in the core gel, fabricate scaffolds in tissue engineering applications, the
followed by release of the drug from the loosened hydrogel real-time monitoring of bone regeneration in vivo remains
Volume 10 Issue 4 (2024) 16 doi: 10.36922/ijb.3448