Page 27 - IJB-10-4
P. 27
International Journal of Bioprinting PAI for 3D bioprinted constructs
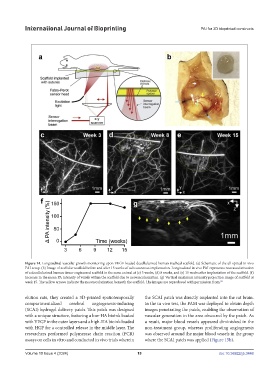
Figure 14. Longitudinal vascular growth monitoring upon VEGF-loaded decellularized human tracheal scaffold. (a) Schematic of the all optical in vivo
PAI setup. (b) Image of acellular scaffold before and after 15 weeks of subcutaneous implantation. Longitudinal in vivo PAI represents neovascularization
of a decellularized human tissue-engineered scaffold in the same animal at (c) 3 weeks, (d) 8 weeks, and (e) 15 weeks after implantation of the scaffold. (f)
Increase in the mean PA intensity of voxels within the scaffold due to neovascularization. (g) Vertical maximum intensity projection image of scaffold at
week 15. The yellow arrows indicate the neovascularization beneath the scaffold. The images are reproduced with permission from. 92
elution rate, they created a 3D-printed spatiotemporally the SCAI patch was directly implanted into the rat brain.
compartmentalized cerebral angiogenesis-inducing In the in vivo test, the PAM was deployed to obtain depth
(SCAI) hydrogel delivery patch. This patch was designed images penetrating the patch, enabling the observation of
with a unique structure, featuring a low-HA bioink loaded vascular generation in the area obscured by the patch. As
with VEGF in the outer layers and a high-HA bioink loaded a result, major blood vessels appeared diminished in the
with HGF for a controlled release in the middle layer. The non-treatment group, whereas proliferating angiogenesis
researchers performed polymerase chain reaction (PCR) was observed around the major blood vessels in the group
assays on cells in vitro and conducted in vivo trials wherein where the SCAI patch was applied (Figure 15b).
Volume 10 Issue 4 (2024) 19 doi: 10.36922/ijb.3448