Page 28 - IJB-10-4
P. 28
International Journal of Bioprinting PAI for 3D bioprinted constructs
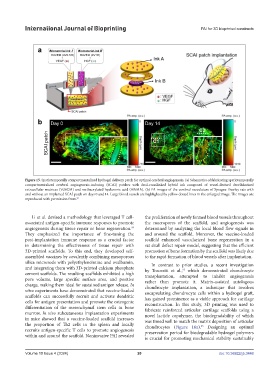
Figure 15. Spatiotemporally compartmentalized hydrogel delivery patch for optimal cerebral angiogenesis. (a) Schematics of fabricating spatiotemporally
compartmentalized cerebral angiogenesis-inducing (SCAI) patches with dual-crosslinked hybrid ink composed of vessel-derived decellularized
extracellular matrices (VdECM) and methacrylated hyaluronic acid (HAMA). (b) PA images of the cerebral vasculature of Sprague-Dawley rats with
and without an implanted SCAI patch on days 0 and 14. Large blood vessels are highlighted by yellow dotted lines in the enlarged image. The images are
reproduced with permission from. 93
Li et al. devised a methodology that leveraged T cell- the proliferation of newly formed blood vessels throughout
associated antigen-specific immune responses to promote the macropores of the scaffold, and angiogenesis was
angiogenesis during tissue repair or bone regeneration. determined by analyzing the local blood flow signals in
94
They emphasized the importance of fine-tuning the and around the scaffold. Moreover, the vaccine-loaded
post-implantation immune response as a crucial factor scaffold enhanced vascularized bone regeneration in a
in determining the effectiveness of tissue repair with rat skull defect repair model, suggesting that the efficient
3D-printed scaffolds. To this end, they developed self- promotion of bone formation by the scaffold was likely due
assembled vaccines by covalently combining mesoporous to the rapid formation of blood vessels after implantation.
silica microrods with polyethyleneimine and ovalbumin, In contrast to prior studies, a recent investigation
and integrating them with 3D-printed calcium phosphate by Tosoratti et al., which demonstrated chondrocyte
95
cement scaffolds. The resulting scaffolds exhibited a high transplantation, attempted to inhibit angiogenesis
pore volume, large specific surface area, and positive rather than promote it. Matrix-assisted autologous
charge, making them ideal for sustained antigen release. In chondrocyte implantation, a technique that involves
vitro experiments have demonstrated that vaccine-loaded encapsulating chondrocyte cells within a hydrogel graft,
scaffolds can successfully recruit and activate dendritic has gained prominence as a viable approach for cartilage
cells for antigen presentation and promote the osteogenic reconstruction. In this study, 3D printing was used to
differentiation of the mesenchymal stem cells in bone fabricate reinforced articular cartilage scaffolds using a
marrow. In vivo subcutaneous implantation experiments novel lactide copolymer, the biodegradability of which
in mice showed that a vaccine-loaded scaffold increases was timed well to match the matrix deposition of articular
the proportion of Th2 cells in the spleen and locally chondrocytes (Figure 16a). Designing an optimal
95
recruits antigen-specific T cells to promote angiogenesis preservation period for biodegradable hydrogel polymers
within and around the scaffold. Noninvasive PAI revealed is crucial for promoting mechanical stability sustainably
Volume 10 Issue 4 (2024) 20 doi: 10.36922/ijb.3448