Page 29 - IJB-10-4
P. 29
International Journal of Bioprinting PAI for 3D bioprinted constructs
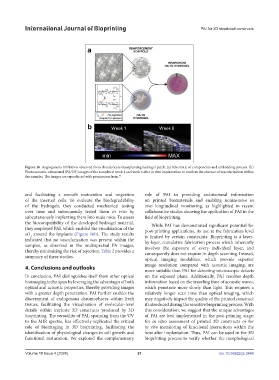
Figure 16. Angiogenesis inhibition observed from chondrocyte transplanting hydrogel patch. (a) Schematic of components and embedding process. (b)
Photoacoustic-ultrasound (PA/US) images of the samples at week 1 and week 6 after in vivo implantation to confirm the absence of vascularization within
the samples. The images are reproduced with permission from. 95
and facilitating a smooth maturation and migration role of PAI in providing architectural information
of the inserted cells. To evaluate the biodegradability on printed biomaterials and enabling noninvasive in
of the hydrogels, they conducted mechanical testing vivo longitudinal monitoring, as highlighted in recent
over time and subsequently tested them in vivo by collaborative studies showing the application of PAI in the
subcutaneously implanting them into nude mice. To assess field of bioprinting.
the biocompatibility of the developed hydrogel material, While PAI has demonstrated significant potential for
they employed PAI, which enabled the visualization of the post-printing applications, its use at the fabrication level
sO around the implants (Figure 16b). The study results is limited by certain constraints. Bioprinting is a layer-
2
indicated that no vascularization was present within the by-layer, cumulative fabrication process which inherently
samples, as observed in the multispectral PA images, involves the exposure of every individual layer, and
thereby minimizing the risk of rejection. Table 2 provides a consequently does not require in-depth scanning. Instead,
summary of these studies. optical imaging modalities, which provide superior
4. Conclusions and outlooks image resolution compared with acoustic imaging, are
more suitable than PAI for detecting microscopic defects
In conclusion, PAI distinguishes itself from other optical on the exposed plane. Additionally, PAI resolves depth
bioimaging techniques by leveraging the advantages of both information based on the traveling time of acoustic waves,
optical and acoustic properties, thereby providing images which penetrate more slowly than light. This requires a
with a greater depth penetration. PAI further enables the relatively longer scan time than optical imaging, which
discernment of endogenous chromophores within fresh may negatively impact the quality of the printed construct
tissues, facilitating the visualization of molecular-level if introduced during the sensitive bioprinting process. With
details within intricate 3D constructs produced by 3D this consideration, we suggest that the unique advantages
bioprinting. The versatility of PAI, spanning from the UV of PAI are best implemented in the post-printing stage:
to the MIR spectra, has effectively replicated the critical for in vitro assessment of printed 3D constructs or for
role of bioimaging in 3D bioprinting, facilitating the in vivo monitoring of functional interactions within the
identification of physiological changes in cell growth and host after implantation. Thus, PAI can be used in the 3D
functional maturation. We explored the complementary bioprinting process to verify whether the morphological
Volume 10 Issue 4 (2024) 21 doi: 10.36922/ijb.3448